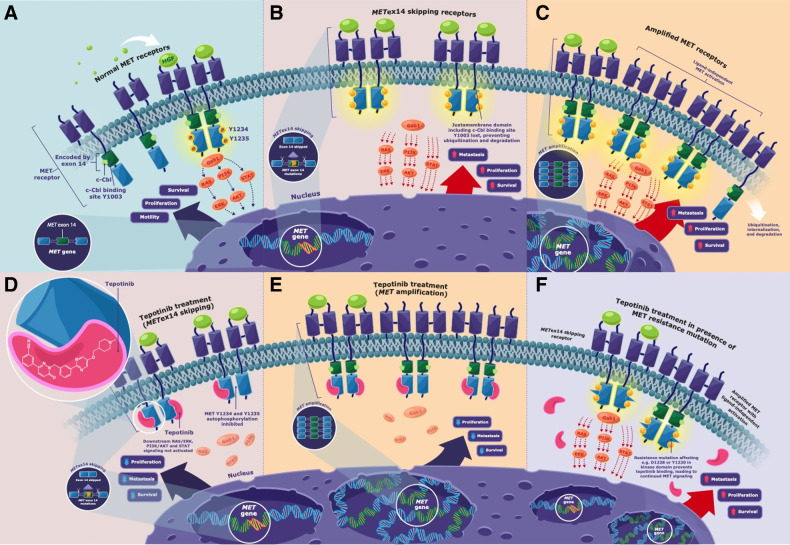
Figure 1.
Simplified diagrammatic schema showing A, In a physiologically normal context, MET signaling is activated when the HGF ligand binds to the extracellular domain of the MET receptor that induces homodimerization and stimulates auto-phosphorylation of the tyrosine residues Y1234 and Y1235 in the cytoplasmic regions of the receptor. This leads to activation and recruitment of the adaptor/scaffold protein Gab1 and activation of downstream signaling pathways (including RAS/ERK, PI3K/AKT, and STAT), resulting in cell survival, proliferation, and motility. Under normal physiological conditions there is a balance between MET signaling and downregulation of MET (3, 4). In addition to MET signaling from the interaction between the ligand and the MET receptor, there have been published reports of MET internalization promoting additional signaling whereby the formation of early MET-containing endosomes can (i) trigger the activation of ERK leading to focal adhesions of the phosphorylated ERK that may mediate HGF-induced cell migration, or (ii) MET may be trafficked along the microtubule network and accumulate in a perinuclear compartment where it may trigger the phosphorylation of STAT3 leading to the translocation of the phosphorylated STAT3 into the nucleus, which could induce downstream signaling (3–5). Publications have also reported the downregulation of MET may occur through (i) endocytosis of MET and the formation of multivesicular bodies leading to MET undergoing lysosomal degradation, or (ii) MET degradation may occur through sequential proteolytic cleavage at the juxtamembrane site, with the cleaved intracellular MET fragment being destroyed in the proteasome, whereas the cleaved extracellular MET may generate an extracellular “decoy MET” that could capture the HGF ligand and interfere with other intact MET receptors (3, 4). B, Dysregulation of the MET pathway can occur through several mechanisms including alterations in the MET gene, such as METex14 skipping (METex14 encodes the MET receptor juxtamembrane domain, which contains negative regulatory elements such as the Y1003-binding site for c-Cbl E3 ubiquitin ligase, which under normal conditions would facilitate the ubiquitination of the MET receptor, resulting in the internalization, trafficking to late endosomes and degradation. However, in tumors harboring METex14 skipping, the receptor is truncated and the loss of this binding site for c-Cbl E3 ubiquitin ligase results in reduced ubiquitination and degradation of the receptor, through decreased lysosomal receptor degradation, leading to sustained MET signaling that can promote uncontrolled proliferation, survival, and metastasis. C, Dysregulation of the MET pathway can also occur through METamp (where the increase in the MET copy number results in increased synthesis of MET, leading to increased MET signaling and subsequent increased cell proliferation, survival, and metastasis). Frazier et al. have reported ligand-independent phosphorylation of receptor tyrosine kinases (RTK) in cancer cells with METamp, where co-localization of MET and RTKs can occur in the Golgi apparatus, and the researchers postulated that when MET is overexpressed, it may accumulate in the Golgi apparatus and this overcrowding facilitates the nonspecific interaction between MET and newly synthesized RTKs (during the RTK trafficking to the plasma membrane) leading to the premature phosphorylation of RTKs and their subsequent downstream effect (5). In tumors harboring METex14 skipping (D) or METamp (E), the MET inhibitor tepotinib binds to the kinase domain and blocks the autophosphorylation of the intracellular domain of the MET receptor, thereby impeding the activation of the downstream signaling pathways (including RAS/ERK, PI3K/AKT, and STAT), and inhibiting tumor cell proliferation, survival, and metastasis. F, Secondary MET kinase domain mutations affecting for example Y1230 and D1228 prevent the binding of tepotinib to the MET receptor, leading to continued MET signaling. AKT, protein kinase B; c-Cbl, Casitas B-lineage lymphoma; ERK, extracellular signal-regulated kinase; Gab1, Grb2-associated binder 1; HGF, hepatocyte growth factor; MET, mesenchymal–epithelial transition proto-oncogene; METamp, MET amplification; METex14, MET exon 14; PI3K, phosphoinositide 3-kinase; RAS, rat sarcoma viral oncogene; STAT, signal transducer and activator of transcription.