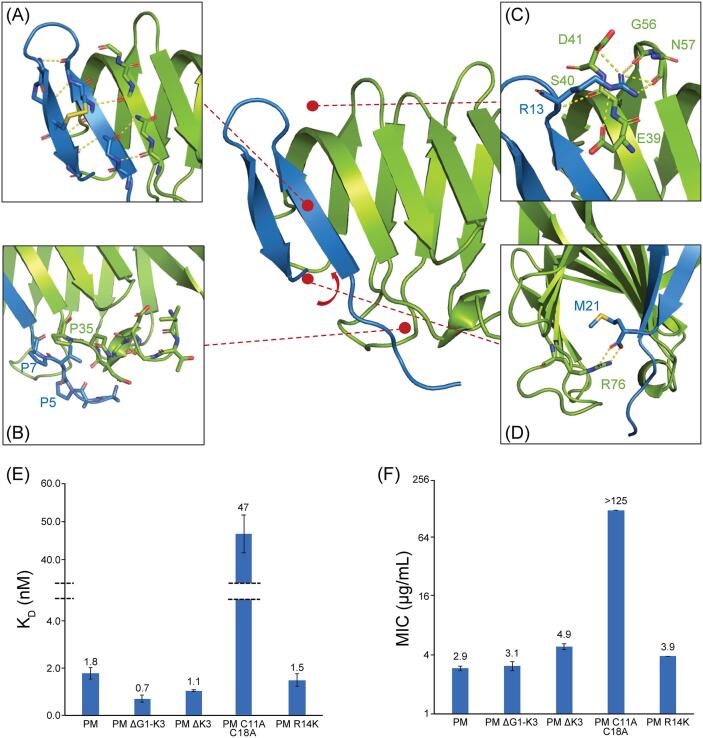
Fig. 4.
Characteristics of P. maculiventris thanatin binding to E. coli LptAm. A, Thanatin binds to the N-terminal β-strand of LptA in a parallel orientation mediated through backbone interactions. Thanatin forms a β-hairpin held together by a disulfide bond and provides a β-strand edge to the complex that disfavors further oligomerization. B, Thanatin contains a flexible N-terminal tail not well defined in the electron density map, thus missing the first several residues and side chains. C, Thanatin R13 of the cationic loop is extensively involved in intermolecular interactions. D, LptA R76 stabilizes the C-terminus of thanatin through bidentate hydrogen bonds. E, Probing importance of shared structural regions by binding. Error bars indicate SEM (minimum of at least n = 3). F, MIC values of thanatin mutants against E. coli ATCC 25922. MIC values are calculated as geometric means, and the error bars represent SEM (minimum of at least n = 3).