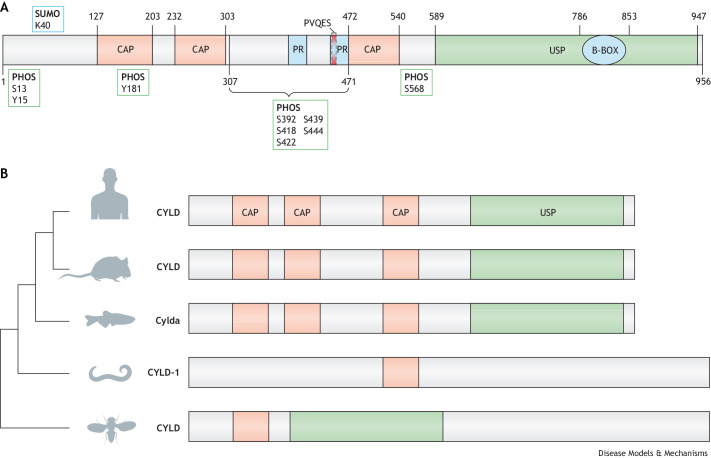
Fig. 2.
CYLD structure, conservation and genetic models. (A) The N-terminal portion of CYLD has three glycine-rich cytoskeleton-associated protein (CAP-Gly) domains. The first, CAP-Gly1 [amino acids (aa) 127-203], is necessary for CYLD association with microtubules (Gao et al., 2008; Yang et al., 2015), while the CAP-Gly3 domain (aa 472-540) binds the nuclear factor kappa-B (NF-κB) essential modifier (NEMO/IKK-γ) that allows modulation of inflammatory NF-κB signalling (Saito et al., 2004). CYLD also contains a TRAF-binding motif (PVQES) (aa 453-457) and two proline-rich motifs (PR) (aa 388-413, 446-471). The C-terminal portion of CYLD contains a zinc-finger like B-box domain (B-BOX; aa 786-853) within the ubiquitin-specific protease (USP) domain. PHOS, phosphorylation sites; SUMO, SUMOylation site. (B) Phylogenetic analysis of CYLD protein and protein domain architecture in human, Mus musculus, Dario rerio, Caenorhabditis elegans and Drosophila melanogaster. CAP, cytoskeleton-associated protein.