Abstract
Background
Few studies have explored the prognostic role of nontraditional lipid-related indicators in non-disabling ischemic cerebrovascular events (NICE). In this study, we aimed to investigate the relationship between the ratio of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol (non-HDL-C/HDL-C) and the1-year risk of recurrent stroke in patients with NICE.
Methods
Total cholesterol (TC), HDL-C, and patient information were collected at admission. Recurrent stroke events were followed up 3, 6, and 12 months after onset. Non-HDL-C levels were calculated by subtracting HDL-C from TC. The non-HDL-C/HDL-C ratio was treated as a continuous variable and in quartiles (Q1–Q4). Stratified multivariate Cox regression was used to investigate the relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke in patients with NICE.
Results
Overall, 1,659 patients with NICE were enrolled. For each unit increase in the non-HDL-C/HDL-C ratio, the 1-year risk of recurrent stroke in patients aged ≥ 65 years (older patients) with NICE increased by 64% in the adjusted model (hazard ratio [HR]: 1.64, 95%confidence interval [CI]:1.18–2.27, P = 0.003), and the HRs were 3.21 and 4.24 times higher in the Q3 and Q4 groups than that in the Q1 group, which was considered to be the reference (adjusted model Q3: HR: 3.21, 95%CI: 1.05–9.83, P = 0.041; adjusted model Q4: HR: 4.24, 95%CI: 1.30–13.85, P = 0.017). However, there was no significant difference in patients younger than 65 years. Both curve fitting and Kaplan–Meier cumulative risk analysis showed that an elevated non-HDL-C/HDL-C ratio significantly increased the 1-year risk of recurrent stroke in older patients with NICE. The optimal range for the non-HDL-C/HDL-C ratio should be no higher than the Q2 group (2.256–2.939). Stratified Cox regression analysis showed that these results tended to be stable for different comorbidities (all P for interaction > 0.05).
Conclusions
Elevated non-HDL-C/HDL-C ratios significantly increased the 1-year risk of recurrent stroke in older patients with NICE. Therefore, clinicians need to pay more attention to this indicator when managing older patients with NICE.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-023-04102-x.
Keywords: Non-disabling ischemic cerebrovascular events, Lipids, Older patients, Recurrent stroke
Background
Non-disabling ischemic cerebrovascular events (NICE), including transient ischemic attack (TIA), minor ischemic stroke, rapid remission, and unimpaired stroke, have attracted significant attention in recent years [1]. The incidence rate of recurrent stroke and cardiovascular events in the first year in patients with NICE is > 6% [1, 2]. Patients with NICE are more likely to be treated aggressively and intensively than those with disabling ischemic cerebrovascular events, thus attracting clinical attention [3, 4].
Previous studies have shown that nontraditional serum lipid-related indicators (including total cholesterol [TC]/high-density lipoprotein cholesterol [HDL-C] ratio, triglycerides [TGs]/HDL-C ratio, and non-HDL-C) are associated with coronary heart disease, asymptomatic intracranial arterial stenosis, and stroke prognosis [5–7]. Non-HDL-C was obtained as a rough measure of TC minus HDL-C and represented the cholesterol content in the atherogenic lipoproteins [3, 8]. The non-HDL-C/HDL-C ratio is obtained by dividing the non-HDL-C levels by the HDL-C levels. This ratio is a nontraditional indicator of dyslipidemia, representing the balance between atherogenic and antiatherogenic lipid particles. It is significantly associated with an increased prevalence of asymptomatic intracranial arterial stenosis [7] and carotid atherosclerosis [9]. In addition, the non-HDL-C/HDL-C ratio is strongly associated with several dyslipidemia-related diseases, such as diabetes mellitus [10], liver function test abnormalities [11], chronic kidney disease [12], and carotid atherosclerosis [9] as well as considered superior to traditional lipid profiles in assessing the degree of arterial stiffness [13].
Many studies have reported that the non-HDL-C/HDL-C ratio is associated with carotid atherosclerosis, intracranial atherosclerotic stenosis, and poor stroke prognosis [5, 14–16]. However, few studies have focused on the relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke in patients with NICE who have a high incidence of recurrent stroke [2, 17]. Therefore, we hypothesized that the non-HDL-C/HDL-C ratio might have a predictive value for recurrent stroke within 1 year in patients with NICE.
Based on the Xi’an Stroke Registry Study platform in China, we collected TC, HDL-C, and other clinical data to investigate the relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke in patients with NICE. We also aimed to explore new cholesterol intervention targets for reducing the 1-year risk of recurrent stroke in patients with NICE.
Methods
Study population
This study is based on 3,117 patients with all stroke subtypes collected in the Xi’an Stroke Registry Study platform from January to December 2015 [18]. These patients were hospitalized for stroke and were followed up at 3, 6, and 12 months after onset. We excluded non-NICE patients, including those with a NIHSS score on admission > 5 (n = 996), and those with hemorrhagic stroke (n = 179). In addition, we also excluded patients who do not have data on non-HDL-C/HDL-C ratio (n = 88), and patients who failed to follow-up at the end of 1 year (n = 195). Finally, a total of 1659 patients with NICE were included. Detailed inclusion and exclusion criteria are presented in Fig. 1. NICE includes minor ischemic stroke, TIA, rapid remission, and unimpaired stroke. Minor ischemic stroke was defined as an NIHSS score on admission ≤ 5 [19]. The TIA was defined as focal brain ischemia with resolution of symptoms within 24 h after onset, which is consistent with the definition used in Wang et al.‘s study [4]. Rapid remission and unimpaired stroke were defined as NIHSS > 5 at the onset & NIHSS 0–5 at arrival. The diagnostic criteria were consistent across all the participating hospitals. Follow-up was conducted from January 2015 to February 2017.
Fig. 1.
Flow chart of the screening and enrollment of study participants. NICE, non-disabling ischemic cerebrovascular events; NIHSS, National Institutes of Health Stroke Scale; HDL-C, high-density lipoprotein cholesterol
This study conforms to the guiding principles of the Declaration of Helsinki. It has been approved by the academic committee of Xi’an No.1 Hospital and the ethics committees of each participating hospital (approval no. 2014 [5]). Written informed consent was obtained from all patients.
Measurements and outcomes
This was a prospective, multicenter, observational cohort study. We obtained baseline data, including demographic information (age, sex, and educational level), cerebrovascular risk factors (including smoking and drinking), and examination on admission (including body mass index [BMI], systolic blood pressure [SBP] on admission, diastolic blood pressure [DBP]on admission, heart rate, NIHSS score on admission). We also obtained previous medical history (including prior stroke, hypertension, diabetes mellitus, and atrial fibrillation) and pneumonia. We looked at laboratory data, including TC, TGs, HDL-C, low-density lipoprotein cholesterol (LDL-C), glycated hemoglobin, fasting blood glucose (FBG), alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, homocysteine, serum creatinine, estimated glomerular filtration rate (eGFR), blood urea nitrogen, uric acid, leukocyte count, and platelet count (Table 1).
Table 1.
Baseline characteristics by non-HDL-C/HDL-C ratio quartiles (Q1-Q4) in patients with NICE.
| Variables | Overall n = 1659 |
Non-HDL-C/HDL-C ratio quartiles | P value | |||
|---|---|---|---|---|---|---|
| Q1, n = 415 | Q2, n = 414 | Q3, n = 415 | Q4, n = 415 | |||
| Demographic information | ||||||
| Age (years) | 64.0 ± 12.1 | 66.3 ± 12.3 | 64.1 ± 12.3 | 64.1 ± 11.2 | 61.5 ± 12.0 | < 0.001 |
| Sex, n(%) | 0.116 | |||||
| male | 1050(63.3) | 266(64.1) | 247(59.7) | 257(61.9) | 280(67.5) | |
| female | 609(36.7) | 149(35.9) | 167(40.3) | 158(38.1) | 135(32.5) | |
| Educational level, n (%) | 0.03 | |||||
| elementary or below | 756(45.6) | 217(52.3) | 180(43.5) | 176(42.4) | 183(44.1) | |
| middle school | 302(18.2) | 67(16.1) | 67(16.2) | 85(20.5) | 83(20) | |
| high school or above | 601(36.2) | 131(31.6) | 167(40.3) | 154(37.1) | 149(35.9) | |
| Cerebrovascular risk factors | ||||||
| Smoking, n (%) | 0.006 | |||||
| Never smoking | 925(55.8) | 218(52.5) | 254(61.4) | 236(56.9) | 217(52.3) | |
| smoking cessation | 318(19.2) | 100(24.1) | 69(16.7) | 77(18.6) | 72(17.3) | |
| current smoking | 416(25.1) | 97(23.4) | 91(22.0) | 102(24.6) | 126(30.4) | |
| Drinking, n (%) | 400(24.1) | 95(22.9) | 81(19.6) | 107(25.8) | 117(28.2) | 0.024 |
| Examination on admission | ||||||
| BMI (kg/m2) | 24.0 ± 3.3 | 23.3 ± 3.6 | 23.8 ± 3.5 | 24.1 ± 3.1 | 24.6 ± 2.9 | < 0.001 |
| SBP on admission (mmHg) | 145.4 ± 20.8 | 144.9 ± 20.8 | 145.8 ± 22.5 | 144.2 ± 19.4 | 146.6 ± 20.4 | 0.363 |
| DBP on admission (mmHg) | 85.5 ± 12.3 | 84.0 ± 11.4 | 85.7 ± 13.2 | 85.1 ± 11.5 | 87.3 ± 12.6 | 0.001 |
| Heart rate (times per minute) | 74.5 ± 9.6 | 74.5 ± 9.8 | 75.0 ± 10.3 | 74.5 ± 8.8 | 73.9 ± 9.5 | 0.424 |
| NIHSS score on admission | 2.0(1.0 ~ 4.0) | 3.0(1.0 ~ 4.0) | 2.0(1.0 ~ 4.0) | 2.0(1.0 ~ 4.0) | 2.0(0.0 ~ 4.0) | 0.226 |
| Previous medical history | ||||||
| Prior stroke, n (%) | 442(26.6) | 117(28.2) | 113(27.3) | 100(24.1) | 112(27) | 0.572 |
| Hypertension, n (%) | 1181(71.2) | 285(68.7) | 294(71) | 292(70.4) | 310(74.7) | 0.271 |
| Diabetes mellitus, n (%) | 378(22.8) | 65(15.7) | 94(22.7) | 88(21.2) | 131(31.6) | < 0.001 |
| Atrial fibrillation, n (%) | 74(4.5) | 20(4.8) | 23(5.6) | 22(5.3) | 9(2.2) | 0.069 |
| Pneumonia, n (%) | 44(2.7) | 12(2.9) | 8(1.9) | 13(3.1) | 11(2.7) | 0.731 |
| Laboratory findings | ||||||
| Total cholesterol (mmol/L) | 4.4 ± 1.1 | 3.7 ± 0.8 | 4.2 ± 0.9 | 4.6 ± 0.9 | 5.1 ± 1.1 | < 0.001 |
| Triglycerides (mmol/L) | 1.7 ± 1.4 | 1.2 ± 0.9 | 1.5 ± 0.9 | 1.7 ± 1.0 | 2.5 ± 2.0 | < 0.001 |
| HDL-cholesterol (mmol/L) | 1.1 ± 0.3 | 1.4 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.2 | 0.9 ± 0.2 | < 0.001 |
| LDL-cholesterol (mmol/L) | 2.6 ± 0.8 | 2.0 ± 0.6 | 2.5 ± 0.7 | 2.8 ± 0.7 | 3.1 ± 0.9 | < 0.001 |
| Glycated hemoglobin,% | 6.4 ± 1.6 | 6.0 ± 1.3 | 6.2 ± 1.5 | 6.4 ± 1.5 | 6.9 ± 1.9 | < 0.001 |
| FBG (mmol/L) | 5.9 ± 2.3 | 5.5 ± 1.8 | 5.7 ± 2.0 | 5.9 ± 2.1 | 6.4 ± 2.8 | < 0.001 |
| Alanine aminotransferase (U/L) | 23.7 ± 18.4 | 23.4 ± 17.8 | 22.5 ± 14.1 | 24.4 ± 23.2 | 24.6 ± 17.4 | 0.343 |
| Aspartate aminotransferase (U/L) | 23.8 ± 12.5 | 24.5 ± 11.7 | 23.9 ± 11.7 | 23.7 ± 15.4 | 23.2 ± 10.9 | 0.482 |
| Alkaline phosphatase (U/L) | 78.3 ± 25.4 | 77.2 ± 28.4 | 79.8 ± 27.1 | 77.8 ± 22.9 | 78.3 ± 22.9 | 0.52 |
| Homocysteine (µmol/mL) | 21.1 ± 14.2 | 19.5 ± 12.7 | 20.5 ± 13.8 | 21.5 ± 15.1 | 23.0 ± 15.0 | 0.035 |
| Serum creatinine (µmol/L) | 75.8 ± 38.5 | 73.3 ± 28.6 | 75.0 ± 42.7 | 74.4 ± 22.4 | 80.4 ± 52.5 | 0.042 |
| eGFR (mL/min/1.73m2) | 75.8 ± 17.7 | 74.1 ± 19.2 | 75.9 ± 18.2 | 76.5 ± 15.1 | 76.7 ± 18.1 | 0.133 |
| Blood urea nitrogen(mmol/L) | 5.0 ± 1.8 | 5.2 ± 2.1 | 5.0 ± 1.7 | 5.0 ± 1.7 | 5.0 ± 1.7 | 0.481 |
| Uric acid(µmol/L) | 292.6 ± 96.5 | 281.9 ± 99.8 | 286.7 ± 95.9 | 294.5 ± 92.6 | 307.2 ± 95.9 | 0.001 |
| Leukocyte count (×109/L) | 6.7 ± 2.3 | 6.4 ± 2.3 | 6.6 ± 2.2 | 6.8 ± 2.3 | 7.0 ± 2.3 | < 0.001 |
| Platelet count (×109/L) | 191.0 ± 59.2 | 179.7 ± 59.9 | 185.7 ± 57.0 | 197.2 ± 58.9 | 201.6 ± 58.6 | < 0.001 |
Notes: data presented are mean ± SD, median (Q1–Q3), or N (%)
Abbreviations: BMI, body mass index; FBG, fasting blood glucose; NIHSS, national institutes of health stroke scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate. Q, quartilesQ1: <2.256, Q2:2.256–2.939,Q3: 2.940–3.749,Q4: ≥3.750
The definition and evaluation criteria of clinically related data in this study were the same as those in the Chinese Intracranial Atherosclerosis Study [20]. Lipid-related indicators, including TC, LDL-C, HDL-C, and TG, were measured through venous blood within 24 h after admission. Non-HDL-C was calculated by subtracting HDL-C from TC. The non-HDL-C/HDL-C ratio was obtained by dividing non-HDL-C by HDL-C levels and was treated as continuous and quartile (Q1–Q4) variables for analysis. Non-HDL-C/HDL-C ratio quartiles (Q1–Q4) were defined as the distribution of non-HDL-C/HDL-C ratio from low to high and divided into four parts according to the quartiles. The ranges of quartiles (Q1–Q4) were as follows: Q1: <2.256, Q2: 2.256–2.939, Q3: 2.940–3.749, and Q4: ≥3.750.
The endpoint event of this study was recurrent stroke within 1 year, defined as the occurrence of new acute stroke events (including acute ischemic stroke, intracranial hemorrhage, subarachnoid hemorrhage, and TIA) during the 1-year follow-up [21]. New acute stroke events were determined by an independent adjudication board composed of stroke specialists from each hospital.
Follow-up
The patients were followed-up at 3, 6, and 12 months after stroke onset, with a time error within 7 days. Data on recurrent stroke were collected via telephone or face-to-face interviews. All follow-up processes were completed by trained research coordinators. For patients with recurrent stroke, the date of the event was recorded. Patients who withdrew from the study or could not be contacted for 5 consecutive working days were considered lost to follow-up.
Statistical analyses
Continuous variables conforming to normal distribution are presented as mean ± standard deviation, and categorical variables are presented as percentages (%). The Chi-square or t-test was used to analyze the differences between the two groups. One-way analysis of variance was used to assess differences among multiple groups of normally distributed variables with consistent variance. Multiple continuous variables among multiple groups with incomplete normal distribution were compared using the Kruskal–Wallis rank-sum test. Multivariate Cox regression models were used to analyze the relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke. The covariates were included as potential confounders based on their relationship with the outcomes of interest or a change in effect estimate of more than 10%.
Smooth curve fitting and Kaplan–Meier (K–M) curves were used to analyze the nonlinear relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke. The log-rank test was used to detect differences in the recurrence curves among quartiles. Subgroup analysis and forest maps were used to analyze the influence of related risk factors and comorbidities on the relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke. All analyses were performed using the statistical software packages R 3.3.2 (http://www.R-project.org, The R Foundation) and EmpowerStats (R) (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA).
Results
Baseline characteristics
At the end of the follow-up period, 195 patients were lost to follow-up. We compared the clinical characteristics of patients lost to follow-up with those who were not lost to follow-up at the end of 1 year. We found that only SBP and DBP at admission significantly differed between the two groups, while the other related clinical characteristics showed no significant difference. Those findings suggest that the patients who were lost to follow-up were close to randomization and had little impact on the relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke (Supplementary Table 1).
The cohort used in our study consisted of 1659 eligible participants with a mean age of 64.0 ± 12.1 years (male: 63.3%). Baseline demographic information, cerebrovascular risk factors, examinations on admission, previous medical histories, and laboratory findings of the non-HDL-C/HDL-C ratio quartiles (Q1–Q4) were compared (Table 1). Patients with a higher non-HDL-C/HDL-C ratio tended to be relatively younger, had higher smoking and drinking rates, lower HDL-C levels, and a higher prevalence of diabetes mellitus, as well as higher education status compared to those with a lower non-HDL-C/HDL-C ratio. In addition, the non-HDL-C/HDL-C ratio was directly proportional to BMI, DBP on admission, TC, TG, LDL-C, glycated hemoglobin, FBG, homocysteine, serum creatinine, and uric acid levels, leukocyte, and platelet counts. The non-HDL-C/HDL-C ratio among quartiles showed no significant differences in terms of sex, SBP on admission, alanine aminotransferase, aspartate aminotransferase, heart rate, NIHSS score on admission, prior stroke, hypertension, atrial fibrillation, pneumonia, alkaline phosphatase, blood urea nitrogen levels, and eGFR.
Cox regression analysis of the non-HDL-C/HDL-C ratio and 1-year risk of recurrent stroke in patients with NICE
In the crude model without adjustments, our results revealed that the Cox regression analysis did not identify a significant association between an increase in the non-HDL-C/HDL-C ratio whether considered as a continuous or categorical variable, and the 1-year risk of recurrent stroke. However, after adjusting for potential confounders in the adjusted model, the 1-year risk of recurrent stroke increased by 43% for each unit increase in the non-HDL-C/HDL-C ratio in patients with NICE (HR: 1.43, 95%CI: 1.11–1.84, P = 0.005). The 1-year risk of recurrent stroke was 2.70-fold higher in Q4 than in Q1, which was considered the reference in patients with NICE (HR: 2.70, 95%CI: 1.06–6.89, P = 0.037) (Table 2). Further analysis revealed that age, as an important confounder, significantly affected the results of the univariate and multivariate Cox regression analyses. Therefore, we performed Cox regression analysis stratified by age < 55, 55–64, 65–74, and ≥ 75 years. The results showed that the 1-year risk of recurrent stroke did not significantly increase with the non-HDL-C/HDL-C ratio in patients aged < 65 years with NICE. The risk significantly increased in ages 65–74 years (HR: 1.92, 95%CI: 1.16 ~ 3.18, P = 0.011) and ages ≥ 75 years (HR: 1.73, 95%CI: 1.04 ~ 2.88, P = 0.036) (Supplementary Table 2).
Table 3.
Multivariate analysis of non-HDL-C/HDL-C ratio and 1-year risk of recurrent stroke in patients with NICE stratified by age of 65 years
| Subgroup | Variable | Overall | Event, n% | Crude Model HR(95%CI) |
P value | Adjusted Model HR(95%CI) |
P value |
|---|---|---|---|---|---|---|---|
| Age < 65 year | |||||||
| Non-HDL-C/HDL-C ratio, per 1 unit increase | 833 | 19(2.3) | 0.91(0.63 ~ 1.32) | 0.61 | 1.23(0.77 ~ 1.95) | 0.391 | |
| Non-HDL-C/HDL-Cratio quartile | |||||||
| Q1 | 178 | 5 (2.8) | Ref. | Ref. | |||
| Q2 | 201 | 6 (3) | 1.08 (0.33 ~ 3.53) | 0.902 | 1.98(0.43 ~ 9.15) | 0.384 | |
| Q3 | 206 | 4 (1.9) | 0.69 (0.19 ~ 2.59) | 0.587 | 1.40(0.26 ~ 7.53) | 0.697 | |
| Q4 | 248 | 4 (1.6) | 0.57 (0.15 ~ 2.13) | 0.406 | 1.36(0.22 ~ 8.37) | 0.744 | |
| P for trend | 0.31 | 0.854 | |||||
| Age≥65 year | |||||||
| Non-HDL-C/HDL-C ratio, per 1 unit increase | 826 | 39(4.7) | 1.33(1.02 ~ 1.74) | 0.038 | 1.64(1.18 ~ 2.27) | 0.003 | |
| Non-HDL-C/HDL-C ratio quartile | |||||||
| Q1 | 237 | 6 (2.5) | Ref. | Ref. | |||
| Q2 | 213 | 9 (4.2) | 1.65 (0.59 ~ 4.64) | 0.341 | 2.02(0.64 ~ 6.39) | 0.229 | |
| Q3 | 209 | 12 (5.7) | 2.26 (0.85 ~ 6.03) | 0.102 | 3.21(1.05 ~ 9.83) | 0.041 | |
| Q4 | 167 | 12 (7.2) | 2.85 (1.07 ~ 7.60) | 0.036 | 4.24(1.3 ~ 13.85) | 0.017 | |
| P for trend | 0.025 | 0.011 |
Notes: Crude adjust none; adjusted for sex; smoking, drinking, prior stroke, pneumonia, NIHSS score at admission, BMI, ALT, triglyceride, FBG, alkaline phosphatase, platelet count, blood urea nitrogen, hypertension, atrial fibrillation, and diabetes mellitus. Abbreviations: BMI, body mass index; FBG, fasting blood glucose; NIHSS, national institutes of health stroke scale; ALT, alanine aminotransferase; HR, hazard ratio; CI, confidence interval; and Q, quartiles. Q1: <2.256, Q2:2.256–2.939, Q3: 2.940–3.749, Q4: ≥3.750
Table 2.
Cox regression analysis of the non-HDL-C/HDL-C ratio and 1-year risk of recurrent stroke in patients with NICE
| Variable | Overall | Event, n% | Crude model HR(95%CI) |
P value | Adjusted model HR(95%CI) |
P value |
|---|---|---|---|---|---|---|
| Non-HDL-C/HDL-C ratio, per 1 unit increase | 1659 | 58(3.5) | 1.09(0.89 ~ 1.33) | 0.425 | 1.43(1.11 ~ 1.84) | 0.005 |
| Non-HDL-C/HDL-C ratio quartile | ||||||
| Q1 | 415 | 11(2.7) | Ref. | Ref. | ||
| Q2 | 414 | 15(3.6) | 1.37(0.63 ~ 2.97) | 0.432 | 1.79(0.75 ~ 4.27) | 0.189 |
| Q3 | 415 | 16(3.9) | 1.45(0.68 ~ 3.13) | 0.339 | 2.27(0.96 ~ 5.38) | 0.063 |
| Q4 | 415 | 16(3.9) | 1.45(0.67 ~ 3.12) | 0.344 | 2.70(1.06 ~ 6.89) | 0.037 |
| P for trend | 0.355 | 0.03 |
Notes: Crude adjust none; adjusted for age, sex; smoking, drinking, prior stroke, pneumonia, NIHSS score at admission, BMI, ALT, triglyceride, FBG, alkaline phosphatase, platelet count, blood urea nitrogen, hypertension, atrial fibrillation, and diabetes mellitus. Abbreviations: BMI, body mass index; FBG, fasting blood glucose; NIHSS, national institutes of health stroke scale; ALT, alanine aminotransferase; HR, hazard ratio; CI, confidence interval; and Q, quartile. Q1: <2.256, Q2:2.256–2.939, Q3: 2.940–3.749, Q4: ≥3.750
Analyzing the relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke in patients with NICE using age-stratified analysis
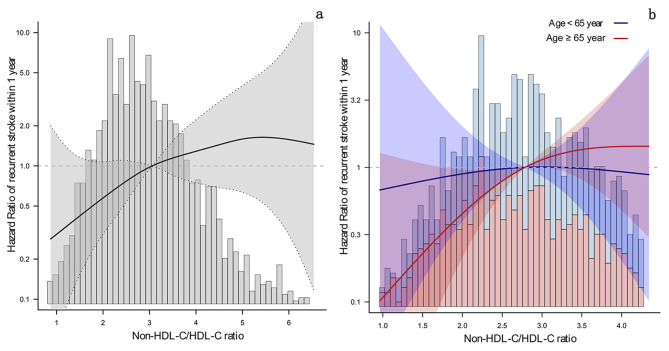
Based on the above analysis, patients with NICE were divided into two groups according to their age, i.e., patients who were aged < 65 years and those aged ≥ 65 years. The results of the Cox regression analysis are shown in Table 3. On the one hand, When treating the non-HDL-C/HDL-C ratio as a continuous variable, for each unit increase of non-HDL-C/HDL-C ratio in patients aged < 65 years with NICE, there were no significant differences in the crude and adjusted models (crude model: HR: 0.91, 95%CI: 0.63–1.32, P = 0.61; adjusted model: HR: 1.23, 95%CI: 0.77–1.95, P = 0.391). However, for older patients (≥ 65 years old) with NICE, the 1-year risk of recurrent stroke increased by 33% in the crude model and 64% in the adjusted model (crude model: HR: 1.33, 95%CI: 1.02–1.74, P = 0.038; adjusted model: HR: 1.64, 95%CI: 1.18–2.27, P = 0.003). The smoothing curve analysis showed an increased linear relationship that went up from left to right between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke in all age groups of NICE patients (Fig. 2a). However, stratified curve fitting showed that this linear relationship was mainly present in older patients (Fig. 2b).
Fig. 2.
The curve fitting of non-HDL-c/HDL-c ratio and 1-year risk of recurrent stroke in patients with NICE. Figure 2a, represents curve fitting regardless of all ages; Fig. 2b, represents curve fitting grouped by age ≥ 65 years. The solid line indicates the adjusted hazard ratio and the dashed lines the 95% confidence interval bands. Age ≥ 65 year were shown in red, and age < 65 years were shown in blue
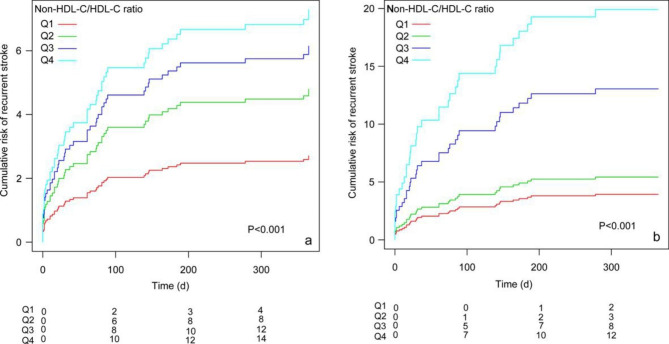
On the other hand, when treating the non-HDL-C /HDL-C ratio as a categorical variable (Q1-Q4). The 1-year risk of recurrent stroke in the Q4 group was 2.85 times higher than that in the Q1 group, which was considered the reference (crude model: HR: 2.85, 95%CI: 1.07–7.60, P = 0.036). The risks of the 1-year risk of recurrent stroke in the Q3 and Q4 groups were 3.21 and 4.24 times higher than in the Q1 group, respectively (adjusted model Q3: HR: 3.21, 95%CI: 1.05–9.83, P = 0.041; adjusted model Q4: HR: 4.24, 95%CI: 1.30–13.85, P = 0.017). Although there was no significant difference between Q2 and Q1, the trend of increased risk was significantly different in the Q1-Q4 groups(P = 0.025 and P = 0.011, respectively).In addition, after adjusting for potential confounding factors (the K-M curve of univariate analysis was illustrated in Supplementary Fig. 1), the K-M cumulative risk analyses also showed that the cumulative risk of the 1-year risk of recurrent stroke increased with an increase in the non-HDL-C/HDL-C ratio from Q1 to Q4 in all age groups (Fig. 3a). The 1-year risk of recurrent stroke was significantly higher in the Q3 and Q4 groups than in the Q1 group, mainly in older patients. The optimal range of the non-HDL-C/HDL-C ratio should be no higher than the Q2 group (2.256–2.939) (Fig. 3b).
Fig. 3.
Cumulative risk of recurrent stroke within 1 year after non-HDL-C/HDL-C ratio as quartiles (Q1-Q4). Figure 3a represents cumulative risk regardless of all ages; Fig. 3b represents cumulative risk grouped by age ≥ 65 years
Subgroups analyses
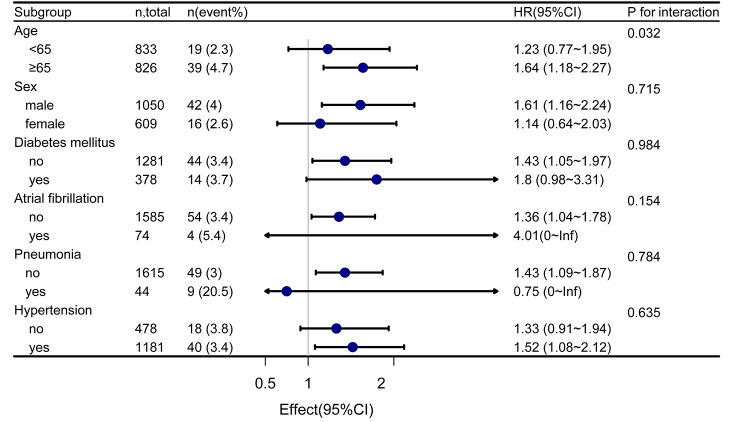
Since stroke patients often have complications that affect the patient’s prognosis, we performed subgroup and interactive analyses to assess whether the relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke was consistent in different subgroups (Fig. 4). The results indicated a significant increase in the 1-year risk of recurrent stroke in NICE patient aged ≥ 65 years with an increase in the non-HDL-C/HDL-C ratio. Furthermore, a significant interaction was observed between patients aged < 65 years and those aged ≥ 65 years (interaction p = 0.032). Although subgroup analysis showed no significant interaction effect in different sexes (P for interaction = 0.715), the 1-year risk of recurrent stroke in men (HR: 1.61, 95%CI: 1.16–2.24) was higher than that in women. Similar results were found in NICE patients without diabetes mellitus (HR: 1.43, 95%CI: 1.05–1.97), without atrial fibrillation (HR: 1.36, 95%CI: 1.04–1.78), and with hypertension (HR: 1.52, 95%CI: 1.08–2.12). There was no significant difference in the interactive analyses.
Fig. 4.
Forest map subgroup analysis of non-HDL-C/HDL-C ratio and 1-year risk of recurrent stroke. Stratified by age, sex, diabetes mellitus, atrial fibrillation, pneumonia, and hypertension
Discussion
Our study showed that the non-HDL-C/HDL-C ratio was an independent risk factor for recurrent stroke within 1 year in patients with NICE. A significant increase in the 1-year risk of recurrent stroke caused by increased non-HDL-C/HDL-C ratio was mainly observed in older patients with NICE but not those aged < 65 years. In the subgroup analysis, the 1-year risk of recurrent stroke increased with an elevated non-HDL-C/HDL-C ratio for men and patients with hypertension, but it was not statistically significant in other comorbidities.
Mounting evidence indicates that nontraditional lipid indicators are associated with stroke prognosis [5, 6]. However, few studies have focused on the relationship between the non-HDL-C/HDL-C ratio and the risk of recurrent stroke, especially in patients with NICE. In this study, our findings indicate that the increased 1-year risk of recurrent stroke was statistically significant only in the adjusted model but not in the crude model (Table 2). After additional covariate screening and stratified analysis, our results demonstrate that age is a crucial factor in the relationship between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke, with 65 years of age identified as the optimal cut-off point (Supplementary Table 2). Specifically, there was a significant increase in the 1-year risk of recurrent stroke associated with an increased non-HDL-C/HDL-C ratio in older patients with NICE, while no such association was observed in those aged < 65 years (Table 3). This result is similar to that of Guo et al.’s, which found that the non-HDL-C/HDL-C ratio as a coefficient of atherosclerosis was associated with intracranial arterial stenosis, a potential risk factor for recurrent stroke [7]. This finding suggests that clinicians should pay attention to the non-HDL-C/HDL-C ratio in older patients with NICE and that early interventions may help reduce the 1-year risk of recurrent stroke.
To explain why the non-HDL-C/HDL-C ratio only significantly increased the 1-year risk of recurrent stroke in age ≥65 years patients with NICE, we conducted a thorough literature review and comparative analysis. The possible explanations are as follows: First, previous studies have shown that the non-HDL-C/HDL-C ratio was significantly associated with an increased prevalence of intracranial arterial stenosis, leading to an increased degree of luminal stenosis, plaque lipid area, intra-plaque hemorrhage, and thrombosis [7, 9, 22, 23].These are independent risk factors for recurrent stroke. Older patients are more susceptible to the effects of the non-HDL-C/HDL-C ratio due to age-related changes in immunity, organ function, blood vessels elasticity, and blood flow velocity, which increase the risk of thrombosis. Second, the proportion of ever smokers was similar across the four groups (Q1-Q4), but the smoking cessation rate was highest in Q1, suggesting that the higher non-HDL/HDL ratio in younger patients may be a reflection of poor health behavior in younger patients. In contrast, older patients in Q4 may have a high non-HDL/HDL ratio despite adequate medical treatment including statin prescription and thus have a higher “true” atherosclerotic burden at baseline. This may be one of the reasons why this indicator only significantly affects stroke recurrence in older patients. Third, some studies have revealed that an elevated non-HDL-C/HDL-C ratio can lead to the occurrence and development of chronic kidney disease and metabolic syndrome [12, 24–26]. Compared with NICE patients aged < 65 years, older patients had lower eGFR levels and higher serum creatinine and blood urea nitrogen levels, suggesting that older patients with NICE are at risk of chronic renal impairment, thus increasing the risk of recurrent stroke. In addition, our results showed that HDL cholesterol, TG levels, and SBP on admission were significantly higher in older patients with NICE than in those aged < 65 years (Supplementary Table 3). Per the criteria for metabolic syndrome [27], the metabolic syndrome risk is significantly increased in older patients with NICE [28]. Fourth, related studies have shown that high non-HDL-C and low HDL-C levels were independent risk factors for mild cognitive impairment [29, 30], more common in older patients with stroke, and associated with atrophy of the hippocampus and recurrent stroke [31–34]. One study showed that approximately 30% of older patients with mild cognitive impairment experience recurrent strokes [33]. These findings suggest that an elevated non-HDL-C/HDL-C ratio (non-HDL-C divided by HDL-C levels) may lead to an increased risk of recurrent stroke by influencing cognitive impairment.
In addition to the above possible explanations, our comparative analysis also found that older patients with NICE have a higher proportion of elementary education background or below, higher NIHSS score on admission, and a significantly higher proportion of prior stroke, pneumonia, and atrial fibrillation than those < 65 years (Supplementary Table 3). These clinical characteristics suggest that, although NICE patients have mild symptoms, older patients with NICE also have more comorbidities associated with an increased risk of recurrent stroke.
To analyze the effect of comorbidities in patients with NICE on our results, we performed a subgroup analysis to explore this issue. There was no statistically significant interaction between the non-HDL-C/HDL-C ratio and the 1-year risk of recurrent stroke in each subgroup. However, the subgroup analysis showed a higher 1-year risk of recurrent stroke in men than in women with NICE (Fig. 4). This finding may be related to the higher proportion of male patients engaged in smoking and drinking and experiencing chronic renal injury (Supplementary Table 4). This sex difference is similar to the results of a previous study that found male patients with high internal carotid artery stenosis had an increased risk of recurrent stroke, mainly due to the high proportion of smoking and high serum creatinine and uric acid levels [35]. Subgroup analysis showed that patients with NICE with hypertension had a higher 1-year risk of recurrent stroke with an elevated non-HDL-C/HDL-C ratio than patients without hypertension. This result is similar to previous studies that found a history of hypertension and elevated SBP after the initial stroke was associated with an increased risk of recurrent stroke [36–38]. Our results also showed that the 1-year risk of recurrent stroke was increased in patients with NICE without diabetes mellitus and atrial fibrillation. However, there are no relevant reports on this aspect. Further analysis of the effect of comorbidities in patients with NICE on the relationship between non-HDL-C/HDL-C ratio and recurrent stroke within 1 year is needed.
Our study has some limitations. First, we only included patients with stroke from four tertiary grade A hospitals in Xi’an, China, which could not represent the status quo of patients with NICE in community hospitals. Second, the imaging indicators related to patients with NICE were not included in our study. Therefore, we did not adjust the indicators, which may have a particular effect on the results. Third, our study did not include the indicators related to the neck, peripheral blood, and intracranial vessels. Finally, since previous studies have not focused on the relationship between the non-HDL-C/HDL-C ratio and recurrent stroke in patients with NICE, our results need to be further verified by subsequent studies.
Conclusions
Our study found that an elevated non-HDL-C/HDL-C ratio significantly increased the 1-year risk of recurrent stroke in older patients with NICE but not in those aged < 65 years. Age was an interaction factor between the non-HDL-C/HDL-C ratio and recurrent stroke. The optimal range of the non-HDL-C/HDL-C ratio should be no higher than Q2 (2.256–2.939). Our results suggest that clinicians assessing older patients with NICE with an appropriate range of non-HDL-C/HDL-C ratios may help reduce the 1-year risk of recurrent stroke.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to all the laboratory technicians, medical staffs and nurses from the participating hospitals. Also, we thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
List of abbreviations
- CI
confidence interval
- BMI
body mass index
- DBP
diastolic blood pressure
- FBG
fasting blood glucose
- eGFR
estimated glomerular filtration rate
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- LDL-C
low-density lipoprotein cholesterol
- NICE
non-disabling ischemic cerebrovascular events
- NIHSS
National Institute of Health Stroke Scale
- non-HDL-C
non-high-density lipoprotein cholesterol
- SBP
systolic blood pressure
- TC
total cholesterol
- TGs
triglycerides
- TIA
transient ischemic attack
Authors’ contributions
SDW, ZZL and XML had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SDW and ZZL planned and designed the study. XML, LXZ, HZ, QLL, WYG, CLH, JW, PL, QQC, MZ, YH, YW and FW contributed to data acquisition and interpretation. ZZL analyzed the data and was primarily responsible for writing the manuscript. LXZ and XML revised the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Funding
This study was supported by the project of Shaanxi Administration of Traditional Chinese Medicine (Grant no. 2022-SLRH-LJ-013); the Science and Technology Program of Shaanxi Province (Grant no.2023-YBSF-041; 2023-YBSF-048;2023-YBSF-052; 2021SF-333; Grant no.2022SF-507; Grant no. 2022SF-381); the Science and Technology Plan Major Project of Xi’an City [Grant no. 201805104YX12SF38(2)]; the Science and Technology Plan Project of Xi’an City [Grant no.22YXYJ0061; 22YXYJ0074;20YXYJ0008(1)]; Scientific research project of Shaanxi Provincial Health Commission [Grant no. 2022C005]; and the Scientific Research Projects of the Xi’an Health Commission (Grant nos. 2020ms03, 2020yb05 2021yb33and 2022qn11). The funders had no role in design and analysis of this trial.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
The study was approved by the academic committee and the ethics committee of Xi’an No. 1 hospital, and was conducted in accordance with the ethical principles for medical research involving human participants as described in the declaration of Helsinki. All patients/participants provided their written and oral informed consents for participating in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhongzhong Liu and Xuemei Lin contributed equally to this work.
References
- 1.Wei-Qi CHEN, Jing MLJING, Yi-Long WANG, Yong-Jun WANG. Why should we focus on non-disabling ischemic cerebrovascular events and its Definition[J] Chin J Stroke. 2018;13(05):470–2. [Google Scholar]
- 2.Amarenco P, Lavallee PC, Labreuche J, Albers GW, Bornstein NM, Canhao P, et al. One-year risk of stroke after transient ischemic attack or minor Stroke[J] N Engl J Med. 2016;374(16):1533–42. doi: 10.1056/NEJMoa1412981. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA et al. /ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines[J]. Circulation, 2019, 139(25):e1082-e1143. [DOI] [PMC free article] [PubMed]
- 4.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack[J] N Engl J Med. 2013;369(1):11–9. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Lee J, Ovbiagele B. Nontraditional serum lipid variables and recurrent stroke risk[J] Stroke. 2014;45(11):3269–74. doi: 10.1161/STROKEAHA.114.006827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Jing J, Wang A, Zhang X, Zhao X, Li Z, et al. Non-high-density lipoprotein cholesterol predicts adverse outcomes in Acute Ischemic Stroke[J] Stroke. 2021;52(6):2035–42. doi: 10.1161/STROKEAHA.120.030783. [DOI] [PubMed] [Google Scholar]
- 7.Guo J, Wang A, Wang Y, Liu X, Zhang X, Wu S, et al. Non-traditional lipid parameters as potential predictors of asymptomatic intracranial arterial Stenosis[J] Front Neurol. 2021;12:679415. doi: 10.3389/fneur.2021.679415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr SS, Hooper AJ, Sullivan DR, Burnett JR. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment[J] Pathology. 2019;51(2):148–54. doi: 10.1016/j.pathol.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Qin G, Tu J, Zhang C, Tang X, Luo L, Wu J, et al. The value of the apoB/apoAIota ratio and the non-HDL-C/HDL-C ratio in predicting carotid atherosclerosis among chinese individuals with metabolic syndrome: a cross-sectional study[J] Lipids Health Dis. 2015;14:24. doi: 10.1186/s12944-015-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N, Hu X, Zhang Q, Bai P, Cai M, Zeng TS, et al. Non-high-density lipoprotein cholesterol: high-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: results from a population-based cohort study[J] J Diabetes. 2018;10(9):708–14. doi: 10.1111/1753-0407.12650. [DOI] [PubMed] [Google Scholar]
- 11.An T, Song Y, Yang Y, Guo M, Liu H, Liu K, et al. Non-HDL-cholesterol to HDL-cholesterol ratio is an independent risk factor for liver function tests abnormalities in geriatric population[J] Lipids Health Dis. 2018;17(1):296. doi: 10.1186/s12944-018-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo PY, Chen XL, Liu YW, Zhang R, He XX, Liu CY. Non-HDL-cholesterol to HDL-cholesterol ratio as an independent risk factor for the development of chronic kidney disease[J] Nutr Metab Cardiovasc Dis. 2015;25(6):582–7. doi: 10.1016/j.numecd.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhao W, Gong W, Wu N, Li Y, Ye K, Lu B, et al. Association of lipid profiles and the ratios with arterial stiffness in middle-aged and elderly Chinese[J] Lipids Health Dis. 2014;13:37. doi: 10.1186/1476-511X-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iannuzzi A, Giallauria F, Gentile M, Rubba P, Covetti G, Bresciani A et al. Association between Non-HDL-C/HDL-C ratio and carotid intima-media thickness in post-menopausal Women[J]. J Clin Med, 2021, 11(1). [DOI] [PMC free article] [PubMed]
- 15.Masson W, Epstein T, Huerin M, Lobo M, Molinero G, Siniawski D. Association between non-HDL-C/HDL-C ratio and carotid atherosclerosis in postmenopausal middle-aged women[J] Climacteric. 2019;22(5):518–22. doi: 10.1080/13697137.2019.1631787. [DOI] [PubMed] [Google Scholar]
- 16.Yang WS, Li R, Shen YQ, Wang XC, Liu QJ, Wang HY, et al. Importance of lipid ratios for predicting intracranial atherosclerotic stenosis[J] Lipids Health Dis. 2020;19(1):160. doi: 10.1186/s12944-020-01336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurford R, Wolters FJ, Li L, Lau KK, Kuker W, Rothwell PM, et al. Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischaemic attack or minor stroke: a population-based cohort study[J] Lancet Neurol. 2020;19(5):413–21. doi: 10.1016/S1474-4422(20)30079-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.W Z F T W. Design and implementation of management system for stroke data mining based on non-structured electronic medical record. [J] China Digital Medicine. 2015;10(2):41–4. [Google Scholar]
- 19.Park HK, Kim BJ, Han MK, Park JM, Kang K, Lee SJ, et al. One-year outcomes after minor stroke or high-risk transient ischemic attack: korean Multicenter Stroke Registry Analysis[J] Stroke. 2017;48(11):2991–8. doi: 10.1161/STROKEAHA.117.018045. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the chinese intracranial atherosclerosis (CICAS) Study[J] Stroke. 2014;45(3):663–9. doi: 10.1161/STROKEAHA.113.003508. [DOI] [PubMed] [Google Scholar]
- 21.Callaly E, Ni Chroinin D, Hannon N, Marnane M, Akijian L, Sheehan O, et al. Rates, Predictors, and outcomes of early and late recurrence after stroke: the North Dublin Population Stroke Study[J] Stroke. 2016;47(1):244–6. doi: 10.1161/STROKEAHA.115.011248. [DOI] [PubMed] [Google Scholar]
- 22.Chen XY, Wong KS, Lam WW, Zhao HL, Ng HK. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study[J] Cerebrovasc Dis. 2008;25(1–2):74–80. doi: 10.1159/000111525. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial Arteries[J] Circ Res. 2017;120(3):502–13. doi: 10.1161/CIRCRESAHA.116.308441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SW, Jee JH, Kim HJ, Jin SM, Suh S, Bae JC, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1[J] Int J Cardiol. 2013;168(3):2678–83. doi: 10.1016/j.ijcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Liu L, Zhang C, Ji S, Mei Z, Li T. Association of metabolic syndrome and its components with risk of Stroke recurrence and mortality: a Meta-analysis[J] Neurology. 2021;97(7):e695–e705. doi: 10.1212/WNL.0000000000012415. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Pan Y, Jing J, Zhao X, Liu L, Meng X et al. Recurrent stroke in minor ischemic stroke or transient ischemic attack with metabolic syndrome and/or diabetes Mellitus[J]. J Am Heart Assoc, 2017, 6(6). [DOI] [PMC free article] [PubMed]
- 27.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement[J] Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 28.Duan Z, Wei X, Liu H, Zhai Y, Hu T, Xu J et al. The effect of metabolic syndrome and/or hyperglycemia on outcomes of acute ischemic stroke patients treated with intravenous thrombolysis[J]. Int J Stroke, 2022:17474930211067352. [DOI] [PubMed]
- 29.Lu D, Li P, Zhou Y, Xu X, Zhang H, Liu L, et al. Association between serum non-high-density lipoprotein cholesterol and cognitive impairment in patients with acute ischemic stroke[J] BMC Neurol. 2016;16(1):154. doi: 10.1186/s12883-016-0668-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, Li Y, Cong L, Hou T, Luo Y, Shi L, et al. High-density lipoprotein cholesterol and brain aging amongst rural-dwelling older adults: a population-based magnetic resonance imaging study[J] Eur J Neurol. 2021;28(9):2882–92. doi: 10.1111/ene.14939. [DOI] [PubMed] [Google Scholar]
- 31.Kwon HS, Lee D, Lee MH, Yu S, Lim JS, Yu KH, et al. Post-stroke cognitive impairment as an independent predictor of ischemic stroke recurrence: PICASSO sub-study[J] J Neurol. 2020;267(3):688–93. doi: 10.1007/s00415-019-09630-4. [DOI] [PubMed] [Google Scholar]
- 32.Qiu C, Zhang Y, Bronge L, Herlitz A, Aspelin P, Backman L, et al. Medial temporal lobe is vulnerable to vascular risk factors in men: a population-based study[J] Eur J Neurol. 2012;19(6):876–83. doi: 10.1111/j.1468-1331.2011.03645.x. [DOI] [PubMed] [Google Scholar]
- 33.D’Souza CE, Greenway MRF, Graff-Radford J, Meschia JF. Cognitive impairment in patients with Stroke[J] Semin Neurol. 2021;41(1):75–84. doi: 10.1055/s-0040-1722217. [DOI] [PubMed] [Google Scholar]
- 34.Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management[J] Ann Transl Med. 2014;2(8):80. doi: 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen CY, Weng WC, Wu CL, Huang WY. Association between gender and stoke recurrence in ischemic stroke patients with high-grade carotid artery stenosis[J] J Clin Neurosci. 2019;67:62–7. doi: 10.1016/j.jocn.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Alter M, Friday G, Lai SM, O’Connell J, Sobel E. Hypertension and risk of stroke recurrence[J] Stroke. 1994;25(8):1605–10. doi: 10.1161/01.STR.25.8.1605. [DOI] [PubMed] [Google Scholar]
- 37.Yuan K, Chen J, Xu P, Zhang X, Gong X, Wu M, et al. A Nomogram for Predicting Stroke recurrence among Young Adults[J] Stroke. 2020;51(6):1865–7. doi: 10.1161/STROKEAHA.120.029740. [DOI] [PubMed] [Google Scholar]
- 38.Friday G, Alter M, Lai SM. Control of hypertension and risk of stroke recurrence[J] Stroke. 2002;33(11):2652–7. doi: 10.1161/01.STR.0000033929.62136.6F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.