Abstract
The diagnostic methods for granting and maintenance of the official tuberculosis-free (OTF) status and for intra-Community movement of cattle are the tuberculin skin tests (single or comparative) and the interferon-γ (IFN-γ) release assay (IGRA). However, until now, IGRAs have been primarily applied in infected farms in parallel to the skin test to maximize the number of infected animals detected. Therefore, an evaluation of the performance of IGRAs in OTF herds to assess whether if their specificity is equal to or higher than that of the skin tests is needed. For this, a panel of 4365 plasma samples coming from 84 OTF herds in six European regions (five countries) was assembled and analysed using two IGRA kits, the ID Screen® Ruminant IFN-g (IDvet) and the Bovigam™ TB Kit (Bovigam). Results were evaluated using different cut-offs, and the impact of herd and animal-level factors on the probability of positivity was assessed using hierarchical Bayesian multivariable logistic regression models. The percentage of reactors ranged from 1.7 to 21.0% (IDvet: S/P ≥ 35%), and 2.1–26.3% (Bovigam: ODbovis–ODPBS ≥ 0.1 and ODbovis–ODavium ≥ 0.1) depending on the region, with Bovigam disclosing more reactors in all regions. The results suggest that specificity of IGRAs can be influenced by the production type, age and region of origin of the animals. Changes in the cut-offs could lead to specificity values above 98–99% in certain OTF populations, but no single cut-off yielding a sufficiently high specificity (equal or higher than that of skin tests) in all populations was identified. Therefore, an exploratory analysis of the baseline IFN-γ reactivity in OTF populations could help to assess the usefulness of this technique when applied for the purpose of maintaining OTF status.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13567-023-01187-5.
Keywords: Bovine tuberculosis, interferon-gamma release assay, specificity, Bayesian statistics, cut-off, Mycobacterium tuberculosis complex
Introduction
Animal tuberculosis is a worldwide zoonotic disease included in the WOAH list of notifiable diseases caused by Mycobacterium tuberculosis complex (MTBC) members [1]. From 2019 to 2022, around 158 countries took measures to prevent animal TB, and 62 of them applied a “test and cull” strategy on their cattle population [2]. This disease affects not only cattle, its main host in most countries, causing bovine tuberculosis (bTB), but also a wide variety of species, both domestic and wild [3, 4]. In the European Union (EU), based on Annex III to Delegated Regulation (EU) 2020/689 and Annex I to Delegated Regulation (EU) 2020/688 of the Regulation (UE) 2016/429, the intradermal tuberculin tests and interferon (IFN)-γ release assay (IGRA) are the official tests for granting and maintenance of the official TB-free (OTF) herd status and to obtain the certification for intra-Community trade of animals.
The IGRA was first introduced in the EU legislation in 2002 for the purpose of maximizing the number of infected animals detected when used in parallel with the skin test [Commission Regulation (EC) No. 1226/2002 of 8 July 2002 amending Annex B to Council Directive 64/432/EEC, both derogated to date]. Since then, several studies have assessed its performance in infected herds, with specificity values (based on Bayesian latent class models) ranging between 62 and 98% (depending on the kit and cut-off applied) [5–12]. In contrast, fewer studies in OTF herds have been performed, suggesting a specificity from 83 to 99% based on the assumption that all reactors were false-positive animals [13–17], and indicating that the IGRA might be a good candidate to be applied under OTF conditions at least in certain cases. However, because different cut-off points, interpretation criteria, kits, and protocols (e.g., time between sample collection and stimulation) were used, comparisons between study results should be interpreted with care.
Given the potential of local factors to influence the specificity of bTB diagnostic tests [15, 18], additional information on the performance of the IGRA test in OTF populations is needed to optimize its use and assess the impact of different cut-off values in the context of maintaining OTF status. For this purpose, an IGRA should ideally offer a specificity not lower than that of the standard test (single or comparative skin tests) while maintaining an adequate (i.e., not lower than that of the standard) sensitivity as specified by the EFSA [19]. However, current estimates of the sensitivity and specificity of IGRAs have been obtained through a range of protocols based on different antigens, subjected to the possible booster effect of a previous skin test, variable times between collection and stimulation of blood samples, different cut-offs, commercial kits, and tests were assayed in different animal populations (in terms of e.g., age, breed, production type, herd size, presence of non-tuberculous mycobacteria (NTM), region, etc.). All these factors may influence the performance of IGRAs [20–25]. Therefore, the assessment of the performance of IGRA tests following harmonised protocols and taking into consideration the potential effect of individual and herd level factors is still needed to assess its suitability for the purpose of granting and maintenance of the OTF status and movement of cattle within the EU [19].
Here, a large panel of samples from five EU countries was assembled and tested using two different IGRA kits, the ID Screen® Ruminant IFN-g (IDvet) and the Bovigam™ TB Kit (Bovigam), in order to i) evaluate the performance of IGRAs under different epidemiological conditions in bovine tuberculosis-free herds with a view to assess its usefulness for granting and maintenance of the OTF status of herds and the intra-Community trade of animals, and ii) assess the impact of different cut-off values in both kits.
Materials and methods
IFN-γ release assay
A panel of 4365 plasma samples coming from six regions (A-F) located in five EU countries (France, Greece, Italy, Romania and Spain) was collected by local authorities and analysed at the European Union Reference Laboratory (EU-RL) for Bovine Tuberculosis located in the Veterinary Health Surveillance Centre (VISAVET) of the Complutense University of Madrid.
Blood samples were collected from OTF herds of animals at least 6 months old. In two regions (B and F) certain OTF herds in which non-specific reactions to the skin test (attributed to the presence of NTM) had previously been described were intentionally included in the study. Samples were collected in heparinized tubes at least 4 months after the previous skin test and transported and stimulated to a laboratory in each of the regions within eight hours post collection. Also, a single skin test was performed the same day the blood sample was collected. Blood from each animal was distributed in four wells of a 24-well plate and stimulated with PBS, avian purified protein derivative (PPDa) (CZ Veterinaria, Porriño, Spain) (20 µg/mL), bovine PPD (PPDb) (CZ Veterinaria, Porriño, Spain) (20 µg/mL) and pokeweed mitogen (Lectin from Phytolacca americana, Sigma, Merck KGaA, Darmstadt, Germany) (2 µg/mL), included as a measure of lymphocyte viability [26, 27]. All antigens and PBS belonging to the same batch were provided by the EU-RL. Plates were then incubated for 18–24 h at 37 °C in a humid atmosphere and then centrifuged at 500–770 g for 10–15 min. Around 400–500 µL of plasma was collected from each well, frozen and shipped to the EU-RL for further analysis.
Plasma-stimulated samples were then analysed for the presence of IFN-γ using the IDvet (ID Screen® Ruminant IFN-γ, IDvet, Innovative Diagnostics, Gravels, France) and Bovigam (Bovigam™ TB Kit, Thermo Fisher Scientific, Waltham, MA, USA) kits in the same day according to the manufacturer instructions (using 25 µL of each sample + 25 µL of dilution buffer 1 for IDvet, and 50 µL of each sample + 50 µL of Green Diluent for Bovigam). Results were expressed as optical densities (OD) by reading the absorbance of each well at 450 nm for IDvet, and at 450 nm with a reference of 620 nm for Bovigam.
For the qualitative interpretation of the Bovigam test two values were considered, the OD of the bovine-stimulated sample (ODbovis) minus the OD of the PBS-stimulated sample (ODPBS), and the ODbovis value minus the OD of the avian-stimulated sample (ODavium).
In the case of the IDvet test, results were transformed to a sample-to-positive (S/P) ratio considering the values of the positive and negative controls included in each plate as follows:
Cut-offs recommended by the manufactures (Table 1) were initially used for interpretation of the quantitative outcomes of the assays.
Table 1.
Cut-off points used for the IFN-γ ELISA assays
| Test | Cut-off | Result | Sample |
|---|---|---|---|
| IDvet | S/P ≥ 35% | Negative | S/P < 35% |
| Positive | S/P ≥ 35% | ||
| Non-valid | S/Pmitogen < 35% or ODPBS > 2.5 or | ||
| ODbovis and ODavium > 2.5 | |||
| Bovigam | ODbovis–ODPBS ≥ 0.1 and ODbovis–ODavium ≥ 0.1 | Negative | ODbovis–ODPBS < 0.1 or |
| ODbovis–ODavium < 0.1 or | |||
| ODbovis–ODPBS ≥ 0.1 and ODbovis–ODavium < 0.1 or | |||
| ODbovis–ODPBS < 0.1 and ODbovis–ODavium ≥ 0.1 | |||
| Positive | ODbovis–ODPBS ≥ 0.1 and ODbovis–ODavium ≥ 0.1 | ||
| Non-valid | ODmitogen–ODPBS < 0.1 or ODPBS > 3.5 or | ||
| ODbovis and ODavium > 3.5 |
In addition, each of the plates were validated considering the following criteria: for IDvet, the mean OD value of the positive controls had to be greater than 0.5 and higher than three times the mean OD value of the negative controls; for Bovigam, the mean OD value of the negative controls had to be below 0.130 with a maximum difference of 0.040 between them, and the mean OD value of the positive controls greater than 0.7 with a maximum difference between them of 30% of their mean value.
Statistical analysis
From each sample, information on the age, region of origin (A–F), production type (beef or dairy), result of the previous cervical skin tests (single or comparative) and the one performed the sampling day (in millimeters), and herd size was available. Previous skin test results could be negative, single-inconclusive [PPDb skin fold increase of ≥ 3 mm but lower than the PPDa skin fold increase and without clinical signs in the inoculation site and therefore negative in the comparative skin test depending on whether herds were subjected to single or comparative skin testing (EU-RL Standard Operating Procedure SOP/001/EURL)]. In addition, for animals coming from regions B and F data on the history of presence of NTM or M. avium subsp. paratuberculosis (MAP) was collected. Also, 510/512 animals in region C were tested using a paratuberculosis (PTB) serology test (ID Screen® Paratuberculosis Indirect ELISA, IDvet, Innovative Diagnostics, Gravels, France).
All statistical analyses were performed in R [28] except where indicated. The proportion of reactors using the default cut-offs was calculated for each test using the default cut-off points (Table 1). The agreement between tests was assessed using the Kappa statistic, the proportion of reactors in each test was compared using the McNemar test and the differences of age between production type was assessed using a Student's t-test. In addition, the quantitative results obtained in the IDvet (S/P ratio) and Bovigam (ODbovis–ODavium) were compared using Pearson’s correlation coefficient.
Then, receiver operating characteristic (ROC) curves were used to evaluate the performance of the IDvet kit at different cut-offs in relation to the qualitative results of the Bovigam with the default cut-off and vice versa. The first analysis (quantitative IDvet results in relation to qualitative Bovigam results) was performed using the R package “pROC” [29]. Confidence intervals (CI) and the optimal cut-off point for the ROC curve was estimated through 1000 bootstrap replicates using the package “cutpointr” [30].
The second analysis (quantitative Bovigam results in relation to qualitative IDvet results) was performed using the package “Epi” [31] to allow the use of two predictors (ODbovis–ODPBS and ODbovis–ODavium) when estimating the ROC curve. Optimal cut-off points were calculated based on the formula:
where “outcome” is the best logistic regression estimate for the optimal cut-off points, β0 is the intercept of the model, β1 and β2 are the coefficients of the predictors, and X1 and X2 the values of the predictors itself.
Finally, the probability of yielding a positive result in the IGRA depending on the effect of the available covariables was evaluated for each kit separately through a Bayesian multivariable logistic regression model of the form:
where Zi,j is the test result (negative/positive) of animal i from herd j, pij is the probability that this animal tests positive, αj is the herd-level effect for herd j, β1, …, βk are the coefficients of the covariables at the animal level, and X1, …, Xk the values of those covariables.
The herd-level effect was then assumed to follow a normal distribution as follows:
where δ1, …, δl are the coefficients of the covariables assessed at herd level and Y1, …, Yl the values of those covariables.
The covariables used at the animal level included the age (available for all animals) and the result of the animal at previous skin tests. The region of origin of the herd, the production type, the herd size and the information on presence of PTB and/or NTM in the herd (yes/no, assuming that animals from herds in which no information on the presence of PTB/NTM was available were not exposed to these bacteria) were included at herd level.
Age and herd size were evaluated alternatively as continuous and categorical variables. For age, four categories were considered: < 1 year, 1–4 years, 4–7 years, and more than 7 years. Herds were categorized based on their size on herds with < 30 animals, 30–59, 60–100, and more than 100 animals.
Samples from region C were subjected to a separate analysis in which the individual result obtained in the PTB serological test was also added as a covariate at the animal level following the same model.
Weakly informative Normal (0, 1) priors were used for the and coefficients. Herd-level random effects (α) were assumed to follow a Normal (μ, σ2) distribution, with σ ~Uniform (0, 1). The best model was selected based on the lowest DIC (Deviance Information Criteria) [32].
Models were fitted in WinBUGS [33] through the R package “R2WinBUGS” [34]. Three Markov Chain Monte Carlo chains were run for 10 000 iterations, with a “burn-in” of 1000 iterations, and posterior distributions were obtained after thinning every 10 iterations. Convergence was assessed visually and more formally using the Gelman-Rubin statistic [35].
Finally, the percentage of reactors at alternative cut-off points within justifiable ranges (S/P ≥ 15–120% range for IDvet, and ODbovis–ODPBS ≥ 0.01–1.0 and ODbovis–ODavium ≥ 0.01–1.0 range for Bovigam) based on the observed quantitative results was assessed to evaluate such thresholds on different populations.
Results
Population of study
All plates were validated according to the manufacturers’ instructions. Out of the 4365 samples received, nine were discarded because there was insufficient volume, and 54 and 49 (~1.5%) yielded non-valid results in the sample stimulated with mitogen when analysed with the IDvet and Bovigam assays, respectively (46 were non-valid in both tests).
Therefore, a total of 4299 samples with results for both tests were included in the study. Animals originated from 84 herds (mean = 51.2 animals per herd, median = 31, range = 5–248), with regions contributing with between 376 and 1225 samples from between 3 and 45 herds (Table 2). All regions included samples from dairy cattle, while beef cattle was not available in regions D and E (Table 2).
Table 2.
Distribution of the population under study
| Region | Total animals | Total herds | Dairy | Beef | ||
|---|---|---|---|---|---|---|
| Animals | Herds | Animals | Herds | |||
| A | 1225 | 11 | 649 | 5 | 576 | 6 |
| B | 1202 | 45 | 607 | 9 | 595 | 36 |
| C | 512 | 8 | 112 | 2 | 400 | 6 |
| D | 495 | 3 | 498 | 3 | – | – |
| E | 376 | 5 | 378 | 5 | – | – |
| F | 489 | 12 | 380 | 10 | 109 | 2 |
| Total | 4299 | 84 | 2619 | 34 | 1680 | 50 |
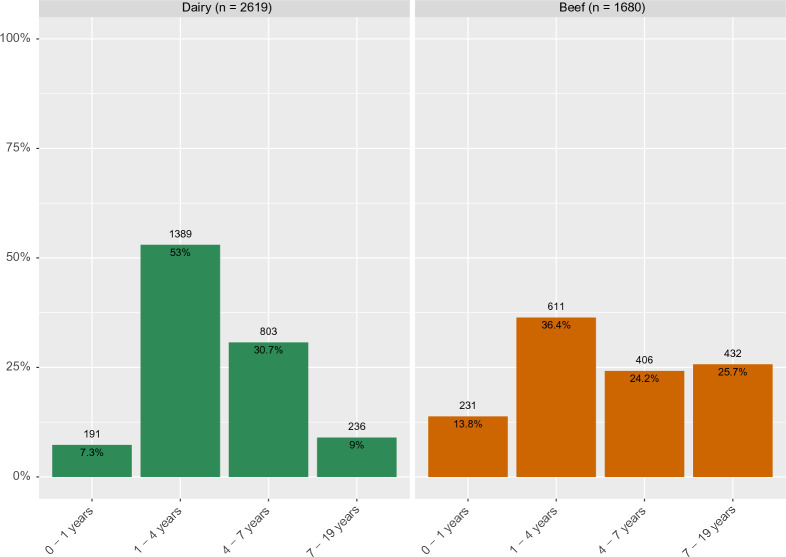
Mean age of sampled animals was 4.2 years (median = 3.6, range = 0.5–18.9), with beef cattle being significantly (Student's t-test, p < 0.001) older (mean = 5.0 years, median = 4.0, range = 0.5–18.9) compared to dairy cattle (mean = 3.8, median = 3.5, range = 0.5–15.9 years) (Figure 1).
Figure 1.
Age of the sampled animals (n = 4299) by production type
Regarding the exposure to other mycobacteria, MAP had been isolated in three and one herds located in regions B (out of 45 herds) and F (out of 12 herds), respectively, and other NTM had been also recovered from cattle located in five herds from region B (in one both MAP and NTM were recovered).
Furthermore, 10 animals from three herds from region C tested positive to the PTB ELISA. Finally, although no reactors were found in the skin test performed when the blood samples were collected, 44/1202 animals from 16/45 herds in region B were comparative-inconclusive, and two out of 489 animals from one herd in region F were single-inconclusive on a previous testing.
Qualitative results using reference cut-off points
A larger proportion of reactors was observed when the Bovigam kit was used compared with the IDvet regardless of the region, production type or age category (overall proportion of reactors in Bovigam 9.8% vs. 7.3% in IDvet, Table 3). Also, there were more herds with at least one positive to Bovigam (60/84 herds; 71.4%) than to IDvet (49/84; 58.3%).
Table 3.
Number of reactors in both kits divided by region, production type and age interval
| Variable | Population | Positive Bovigama | Positive IDvetb | |||
|---|---|---|---|---|---|---|
| Animals | Herds | Animals | Herds | Animals | Herds | |
| Region | ||||||
| A | 1225 | 11 | 26 (2.1%) | 7 (63.6%) | 21 (1.7%) | 6 (54.5%) |
| B | 1202 | 45 | 104 (8.7%) | 27 (60%) | 71 (5.9%) | 19 (35.2%) |
| C | 512 | 8 | 43 (8.4%) | 7 (87.5%) | 32 (6.3%) | 6 (75%) |
| D | 495 | 3 | 45 (9.1%) | 3 (100%) | 28 (5.7%) | 3 (100%) |
| E | 376 | 5 | 99 (26.3%) | 5 (100%) | 79 (21.0%) | 5 (100%) |
| F | 489 | 12 | 106 (21.7%) | 11 (91.7%) | 84 (17.2%) | 10 (83.3%) |
| Production type | ||||||
| Dairy | 2619 | 34 | 345 (13.2%) | 34 (100%) | 265 (10.1%) | 32 (94.1%) |
| Beef | 1680 | 50 | 78 (4.6%) | 26 (52%) | 50 (3.0%) | 17 (34%) |
| Age interval | ||||||
| < 1 year | 422 | 64 | 42 (10.0%) | 26 (40.6%) | 28 (6.6%) | 19 (29.7%) |
| 1–4 years | 2000 | 82 | 246 (12.3%) | 47 (57.3%) | 189 (9.5%) | 38 (46.3%) |
| 4–7 years | 1209 | 78 | 106 (8.8%) | 32 (41.0%) | 71 (5.9%) | 23 (29.5%) |
| > 7 years | 668 | 72 | 29 (4.3%) | 17 (37.5%) | 27 (4.0%) | 16 (22.2%) |
| Herd size | ||||||
| < 30 animals | 672 | 41 | 63 (9.4%) | 22 (53.7%) | 40 (5.6%) | 15 (36.6%) |
| 30–60 animals | 78 | 18 | 125 (16.0%) | 17 (94.4%) | 97 (12.4%) | 14 (77.8%) |
| 60–100 animals | 891 | 12 | 133 (15.0%) | 10 (83.3%) | 108 (12.1%) | 10 (83.3%) |
| > 100 animals | 1955 | 13 | 102 (5.2%) | 11 (84.6%) | 70 (3.6%) | 10 (76.9%) |
| NTM/PTB presencec | ||||||
| Yes | 524 | 8 | 63 (12.0%) | 7 (87.5%) | 52 (9.9%) | 6 (75%) |
| Total | 4299 | 84 | 423 (9.8%) | 60 (71.4%) | 315 (7.3%) | 49 (58.3%) |
aODbovis–ODPBS ≥ 0.1 and ODbovis–ODavium ≥ 0.1
bS/P ≥ 35%
cHerds without notification of NTM/PTB where not considered in the table
The lowest number of reactors was found in region A (2.1% for Bovigam and 1.7% for IDvet); regions B, C and D yielded a similar proportion of positive animals (ranging between 8.4–9.1% for Bovigam and 5.7–6.3% for IDvet), while the highest number of reactors was observed among samples collected from regions E and F (> 17% for both kits) (Table 3).
Dairy animals were more likely to test positive, with 2.9 and 3.4 times more reactors compared with beef cattle when considering the Bovigam and IDvet kits, respectively (Table 3). Likewise, at least one positive result to Bovigam and IDvet was found in all and 32/34 dairy herds, respectively, compared with 26/50 and 17/50 beef herds with at least one positive for Bovigam and IDvet, respectively. Finally, more reactors were also observed among animals from 1 to 4 years while fewer were found among older (> 7 years) animals irrespective of the kit (Table 3).
In addition, all 10 reactors to the PTB ELISA were negative to both IGRA kits. Also, of the 44 comparative-inconclusive animals in previous skin tests, only three animals from three herds and five animals from five herds were positive to IDvet and Bovigam, respectively, and there was only one positive to Bovigam out of the two inconclusive animals in region F.
Agreement and correlation between test results
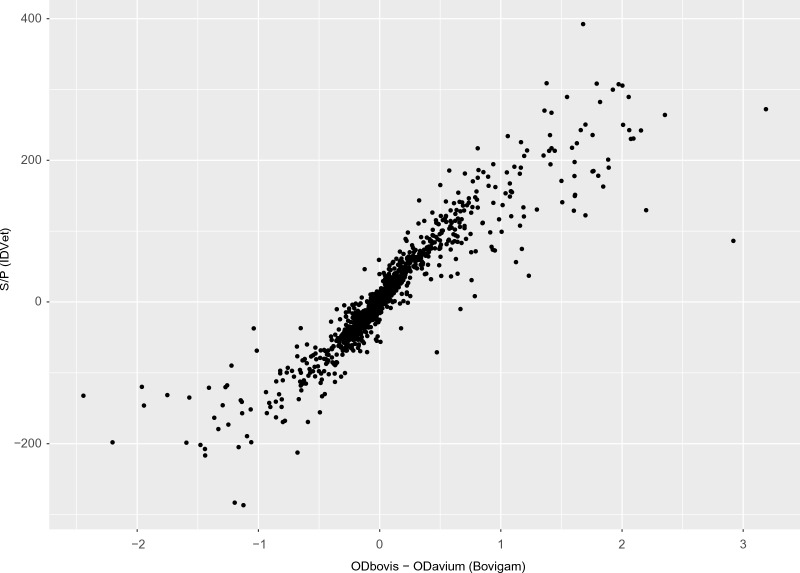
When the quantitative results obtained in both tests were compared, a high correlation between the S/P ratio (IDvet) and the difference between bovine and avian OD values (Bovigam) was observed (0.919, 95% CI 0. 914–0.923) (Figure 2).
Figure 2.
Quantitative results for each of the samples analysed for both kits
The agreement between the qualitative results obtained using the default cut-offs was moderate considering both tests aim at the same target (Kappa = 0.80; 95% CI 0.76–0.83) with a significantly (McNemar test, p < 0.001) larger proportion of animals positive only to the Bovigam kit (Table 4).
Table 4.
Agreement between the results obtained in Bovigam and IDvet IFN-γ kit at default cut-off points
| IDvet (S/P ≥ 35%) | ||||
|---|---|---|---|---|
| Negative | Positive | Total | ||
| Bovigam (ODbovis–ODPBS ≥ 0.1 and ODbovis–ODavium ≥ 0.1) | Negative | 3861 | 15 | 3876 |
| Positive | 123 | 300 | 423 | |
| Total | 3984 | 315 | 4299 | |
ROC analysis
The ROC analysis of the quantitative S/P values from IDvet using the qualitative results in the Bovigam kit as a reference yielded a high value of the Area Under the Curve (AUC) (0.984, 95% CI 0.975–0.992) with an optimal cut-off point of 15.175, leading to a sensitivity of 96.7% and specificity of 96.1% (Figure 3). The impact of using alternative cut-offs in the interpretation of the IDvet results in the sensitivity and specificity of the test with regards to the Bovigam results is shown in Additional file 1.
Figure 3.
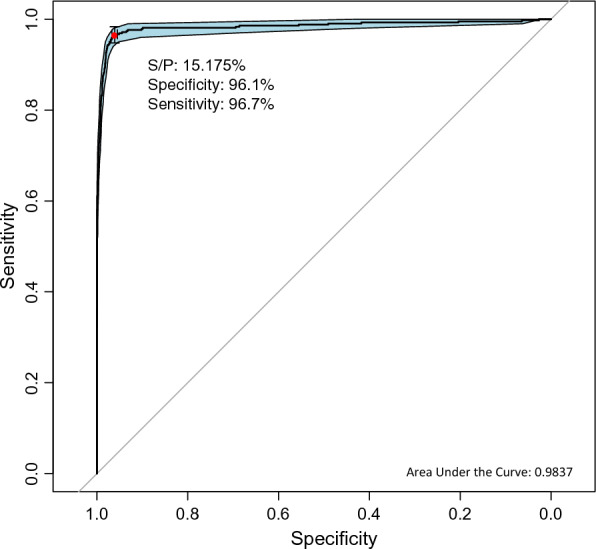
ROC curve of the performance of the IDvet kit against the result of Bovigam kit. Red dot represents the optimal cut-off point for maximum specificity and sensitivity (S/P = 15.175%) along with the specificity (96.1%) and sensitivity (96.7%)
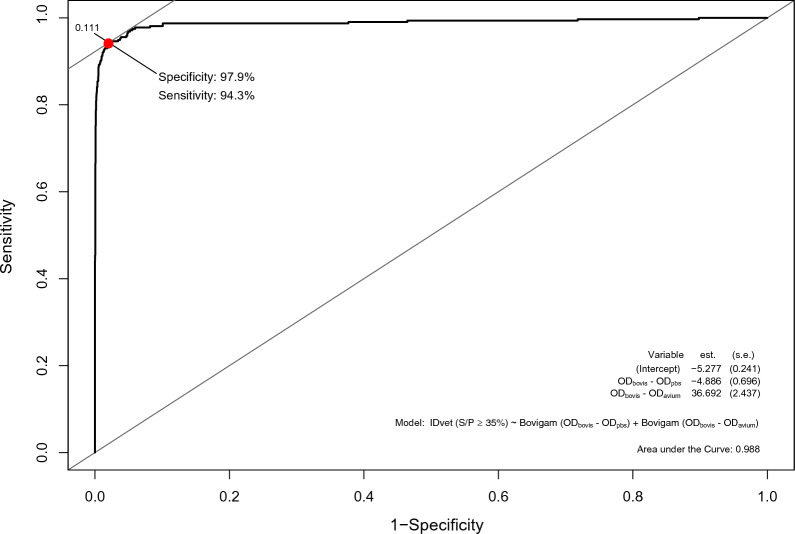
Likewise, the analysis of the quantitative Bovigam values (ODbovis–ODPBS and ODbovis–ODavium) using the qualitative IDvet results as the reference revealed a high AUC value (0.988, 95% CI 0.986–0.990) with the optimal cut-off points identified, yielding a Se of 94.3% and a Sp of 97.9% (Figure 4). Additional information on the impact of other cut-offs in the Se and Sp of the Bovigam test is shown in Additional file 1.
Figure 4.
ROC curve of the performance of the Bovigam kit against the result of IDvet kit. On the bottom-left are represented the model coefficients for ODbovis–ODPBS and ODbovis–ODavium. Red dot represents the logistic regression estimate for the optimal cut-off points for maximum specificity and sensitivity along with the specificity (97.9%) and sensitivity (94.3%). Optimal cut-off points are shown in Additional file 1
Multivariable regression
The final model for both kits included the age, production type and region (Table 5).
Table 5.
Estimates of the association of covariables with positivity according to the Bayesian logistic regression models
| Variables | Bovigam (ODbovis–ODPBS ≥ 0.1 and ODbovis–ODavium ≥ 0.1) | IDvet (S/P ≥ 35%) | ||||
|---|---|---|---|---|---|---|
| Median | 95% PPIa | Median | 95% PPIa | |||
| 2.5% | 97.5% | 2.5% | 97.5% | |||
| Regionb | ||||||
| A | 1 | – | – | 1 | – | – |
| B | 5.4 | 2.7 | 11.5 | 4.6 | 1.8 | 12.4 |
| C | 9.7 | 3.9 | 25.4 | 14.2 | 4.3 | 51.4 |
| D | 3.9 | 1.3 | 11.4 | 2.7 | 0.7 | 11.3 |
| E | 11.4 | 4.6 | 28.7 | 10.9 | 3.4 | 36.4 |
| F | 11.0 | 5.1 | 24.3 | 9.6 | 3.5 | 26.7 |
| Production typeb | ||||||
| Beef | 1 | – | – | 1 | – | – |
| Dairy | 3.7 | 2.1 | 6.7 | 6.3 | 3.0 | 14.3 |
| Agec | ||||||
| > 7 years | 1 | – | – | 1 | – | – |
| < 1 year | 3.4 | 2.0 | 5.9 | 2.4 | 1.3 | 4.4 |
| 1–4 years | 2.4 | 1.5 | 3.7 | 1.8 | 1.1 | 2.8 |
| 4–7 years | 1.5 | 0.96 | 2.4 | 0.95 | 0.6 | 1.6 |
aPosterior probability interval
bHerd level
cAnimal level
The region was strongly associated with the probability of testing positive to the test, with animals from all regions but D having a higher probability of being a reactor compared with the reference region (A) (Table 5). In addition, odds of positivity in dairy cattle were 3.7 (95% posterior probability interval (PPI): 2.1–6.7) and 6.3 (95% PPI: 3.0–14.3) higher than in beef cattle to the Bovigam and IDvet test, respectively (Table 5).
Finally, younger animals (< 1–4 years) had higher odds of being positive compared to older animals irrespective of the kit used (Table 5).
Assessment of alternative cut-off points
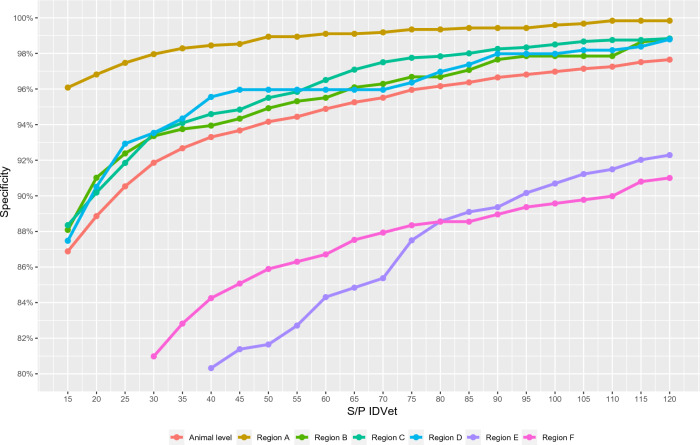
To assess the potential impact of using different cut-offs, the proportion of reactors observed when the cut-off was set at any point in the S/P ≥ 15–120% range (IDvet) and ODbovis–ODPBS ≥ 0.05–1.0 and ODbovis–ODavium ≥ 0.05–1.0 (Bovigam) was calculated. A perfect specificity (i.e., no reactors) was not achieved in any region regardless of the cut-off point in the ranges considered for both kits, except if we consider beef population from region A, in which a 100% specificity was achieved at a S/P ≥ 60% (IDvet) and at ODbovis–ODPBS ≥ 0.2 and ODbovis–ODavium ≥ 0.5 (Bovigam) (Figure 5 and Additional file 2). Furthermore, the proportion of reactors at the different cut-off values considered varied largely depending on the region (Figure 5), and for those regions in beef and dairy cattle were tested, depending on the production type within a region (see Additional file 2).
Figure 5.
Variation of the animal-level specificity depending on the cut-off point for IDvet (S/P ratio). Red line represents the global animal-level specificity while the others represent region animal-level specificity
Discussion
The great efforts invested for decades in surveillance, control and eradication programs in many countries have led to the achievement of OTF status in multiple regions and countries [36, 37]. However, in order to maintain such disease-free status, continuous monitoring is still required. In this context, the use of tests that have an optimal specificity (while maintaining an adequate sensitivity) is of paramount importance to avoid false-positive results, which could occur even with very specific tests when applied to large populations. In Europe, the single and comparative skin tests have been routinely used for this purpose, yielding excellent results in terms of specificity in the majority of the cases [38, 39]. Nevertheless, the numerous limitations associated with their use related to difficulties in their standardization (due to practical constrains in the field, the inherent subjectivity interpreting the test, and other factors linked with the test itself) [40, 41] have led to the consideration of IGRAs as an alternative for granting and maintenance of the OTF status of herds and for the intra-Community trade of animals [19]. The use of IGRAs would solve certain practical issues, since they only require a single visit to the farm, and most of the IGRA protocol is conducted in the laboratory, where conditions are easier to standardize [42]. Still, certain factors can still affect its performance [15, 18, 20], among which the cut-off value for interpretation is a major issue.
Because IGRAs in Europe have been mostly applied in bTB-infected herds, cut-off points used routinely in the EU have been typically evaluated in terms of their usefulness to maximize the diagnostic sensitivity when used in parallel to the skin tests [12, 43]. Furthermore, the specificity of IGRAs in that situation has been sometimes criticized, with most estimates suggesting it may be considerably lower than that of skin tests, although this would be also highly dependent (in addition to the cut-off) on the antigens used [23, 44, 45] and the animal populations tested [17, 18]. In this study, we aimed at assessing the performance of IGRA in OTF populations using the cut-offs currently recommended by the manufacturers on cattle populations from different regions and production types while standardizing as much as possible the protocol in order to minimize the possible impact of factors associated with the test.
Only five studies have assessed the performance of IGRAs in OTF populations, of which two were published over 15 years ago and four considered only the Bovigam kit [13–17, 22]. Overall, Bovigam specificity values obtained here were similar to previously estimated, with values around 90% despite considering different cut-offs and protocols, except for Keck et al. [17] where a 99.9% specificity was observed on bullfighting cattle, a population not evaluated here that is known to have a lower IFN-γ production [46], and for Faye et al. [22] for which depending on the interpretation criteria a 97.6–99.4% specificity was observed. In contrast, for IDvet, evaluated in OTF herds in only one study [16], previous specificity estimates were higher than the ones observed here for the overall population but very similar to those from region A, with values around 98%.
The diagnostic specificity of Bovigam and IDvet kits has been simultaneously assessed in only two studies (one in OTF herds and one in infected herds), both suggesting that the use of Bovigam would result in a higher number of reactors compared to IDvet [12, 16], similar to what was observed here (Table 3). Despite these results, the probability of yielding a positive outcome for both tests at default cut-offs was influenced by the same variables (Table 5) and, as shown by the ROC analysis from this study (Figures. 3, 4) and previous results from infected herds [12], both tests behaved similarly. Overall, this suggests that both tests are subjected to a similar effect of external variables, and that part of the differences in their performance observed here are derived from the application non-equivalent cut-off points rather than from factors such as the use of twice fold more plasma for Bovigam than IDvet, considering that both tests were performed using same PPDs, so the disparities in terms of diagnostic accuracy might not be as high as proposed between kits [47].
The influence of production type and age on the increase of the probability of observing a (false) positive result in the test identified here agrees with previous studies: dairy cattle were also more prone to yield IGRA positive results compared to beef in a previous study conducted in Italy [15], what could be attributed to exposure to other infections more prevalent in dairy animals like PTB, leading to an increased amount of non-specific immune reactions [48].
Also, we found that the risk of positivity decreased with age, with animals of < 1 year having the higher odds, as suggested by Keck et al. [17]. In contrast, this was different from the lack of an age-associated risk described in Cagiola et al. in an OTF population, although only animals between 2 and 6 years were considered there [15]. Furthermore, an increase in the risk of positivity with increasing age was suggested in another study when comparing animals of ≥ 3 years with those < 1 year [18]. Altogether, this suggests that the direction of the age effect may be different depending on local factors. For example, in our study there were no reactors of < 1 year in region D to any of the tests, and less compared to ≥ 7 years animals in dairy herds from region B (see Additional file 3). Despite this, the model indicates that < 1 year old animals have 3.4 and 2.4 more risk of positivity than older animals (for Bovigam and IDvet, respectively), what could be related to a higher non-specific IFN-γ production mediated by NK cells in younger cattle [49, 50], limiting its use in calves < 6 months old in the EU (EU-RL Standard Operating Procedure SOP/004/EURL and SOP/006/EURL).
Neither herd size nor the presence of NTM or PTB were included in the final model. Regarding the former, herd size did not influence the individual risk of being positive to any of the tests, suggesting that practices associated with larger herds (e.g., more animal movements and contacts between animals) may not play a role in such effect once other local factors are taken into account.
False-positive reactions in all bTB tests (including IGRAs) have been linked to the presence of NTM and/or PTB [51–53]. We did not find evidence of this association, but this result should be interpreted with care since herds were not subjected to a systematic evaluation of the presence of NTM or PTB; therefore, even though this variable was not included in the final model, the presence of NTM/PTB as a possible source of false-positive reactions should be further considered, and even more considering the higher prevalence of these types of infections in dairy cattle [48], which was found to play an important role in the risk of an animal testing positive for IGRA. In this sense, the use of defined MTBC-mycobacterial antigens (e.g., ESAT6, CFP10, Rv-3615c) could be useful to minimize cross-reactions in the IGRA due to NTM/PTB [22, 45, 54].
As stated before, region had a strong influence on the risk of positivity; this was also evident when changes in the proportion of reactors depending on the cut-off applied for each region were assessed (Figure 5): in certain regions (particularly region A) the use of IGRA in OTF herds could lead to high specificity values (> 98%) at cut-offs below S/P = 35%, while this could not be achieved in others (E and F) even when considering cut-offs that would most likely lead to unacceptable diagnostic sensitivities. Interestingly, these differences were observed despite using the same tuberculin for stimulation of all the blood samples regardless of their origin, thus removing the variability associated with the use of different tuberculins in different countries, a well-known factor influencing bTB diagnostic performance [55]. Season could influence the performance of the IFN-γ due to the possible impact of environmental conditions on the viability of the samples [21, 56] and the occurrence of non-specific immunological stimuli [57]. All samples were collected between November and February except those from region E, in which animals were sampled between May and June. Therefore, although a possible effect of the environmental conditions cannot be ruled out (particularly for region E), this is unlikely to explain the wide variation observed in the proportion of reactors depending on the region. Overall, no single (usable) cut-off that would yield the same specificity across populations was identified, a key aspect for its harmonisation at the EU level [19]. In this context and considering the widely different results obtained in the different regions, it would be advisable to establish the baseline reactivity of OTF populations before the implementation of the IGRA as a routine test for maintenance of the OTF status.
The use of IGRA has several advantages over the skin test, the main one being the application of an objective criteria for interpretation of the results, thus minimizing possible biases associated with external factors that can hamper accurate skinfold thickness measurements. However, in light of our results, serial application of the single or comparative skin test in animals testing positive to IGRA could help to ensure an adequate specificity if overall sensitivity is ensured, while maximizing these practical advantages.
The proportion of reactors found when using both IGRA kits evaluated here was highly dependent on the population tested, and results obtained in both kits were influenced by the age and production type of the animals to a similar degree. When considering the quantitative results both kits performed similarly, suggesting that the differences in the proportion of reactors (higher in the case of Bovigam compared to IDvet) were partly due to the use of non-equivalent cut-offs. Based on the information presented here, IGRAs may be considered a reliable alternative to skin tests in certain populations for granting and maintenance of the OTF status and movement of cattle within the EU, but no single cut-off yielded a sufficiently high specificity in all OTF populations evaluated here. Therefore, a careful preliminary assessment of the baseline IGRA reactivity in OTF populations before its application, and the possible use of other tests contemplated in the legislation (i.e., the single or comparative skin test) applied in series to IGRAs in certain epidemiological scenarios so that the overall sensitivity is not compromised, can help to ensure its adequate performance.
Supplementary Information
Additional file 1: Impact of different cut-offs for Bovigam and IDvet in the sensitivity and specificity of the opposite test.
Additional file 2: Evolution of the animal-level specificity of the Bovigam and IDvet kit depending on the cut-off point (OD or S/P ratio) used to define positivity, depending on different covariables.
Additional file 3: Distribution of reactors depending on the age by region and production type.
Acknowledgements
The authors would like to thank the collaboration and support of the EU-RL for Bovine Tuberculosis, the Spanish Ministry of Agriculture, Fisheries and Food, to the Consejería de Agricultura, Ganadería y Desarrollo Rural of the Junta de Castilla y León and the Consellería do Medio Rural of the Xunta de Galicia, as well as the French Ministry of Agriculture, the veterinary regional laboratory of Côte d’Or and the National Reference Laboratories for Bovine Tuberculosis from Greece, Italy and Romania. Also, we would like to thank Francisco Lozano, Cristina Viñolo, Laura Sánchez Martín and Marta Almeida Santiago from the Mycobacteria Unit of VISAVET Health Surveillance Centre for their technical support.
Authors' contributions
BR, JB, LDJ and JA planned and designed the experiments. JLS, IA, MLP, MLB, SG, EG, FB, NK and AS participated on sample collection. AGB performed the laboratory analysis and collected the data. AGB and JA performed the statistical analyses and drafted the first version of the manuscript. All authors read and approved the final manuscript.
Funding
AGB holds a PhD fellowship (reference CT58/21-CT59/21) from the Universidad Complutense de Madrid and Banco Santander. JA is the recipient of a Ramón y Cajal contract from the Spanish Ministry of Economy, Industry and Competitiveness (MINECO, RYC-2016-20422). This work was funded by the European Union Reference Laboratory for Bovine Tuberculosis and by the National Reference Laboratories for Bovine Tuberculosis from France, Greece, Italy and Romania, and is a contribution to the project Integrated Strategies for Tuberculosis Control and Eradication in Spain (ERATUB) (RTI2018-096010-B-C22, Ministerio de Ciencia, Innovación y Universidades, MICINN).
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WOAH (2022) Infection with Mycobacterium tuberculosis complex. In: Terrestrial animal health code. https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_bovine_tuberculosis.htm
- 2.WOAH (2023) OIE-WAHIS. https://wahis.woah.org/#/dashboards/control-measure-dashboard. Accessed 1 Mar 2023
- 3.Pesciaroli M, Alvarez J, Boniotti MB, Cagiola M, di Marco V, Marianelli C, Pacciarini M, Pasquali P. Tuberculosis in domestic animal species. Res Vet Sci. 2014;97:78–85. doi: 10.1016/j.rvsc.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Bezos J, Álvarez J, Romero B, de Juan L, Domínguez L. Bovine tuberculosis: historical perspective. Res Vet Sci. 2003;97:S3–4. doi: 10.1016/j.rvsc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mouqatea S, Alkhamis M, Akbar B, Ali A, Al-Aqeel H, Bin-Heji A, Razzaque M, Alvarez J, Perez A. Bayesian estimation of ELISA and gamma interferon test accuracy for the detection of bovine tuberculosis in caudal fold test-negative dairy cattle in Kuwait. J Vet Diagn Invest. 2018;30:468–470. doi: 10.1177/1040638718759574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahuerta-Marin A, Milne MG, McNair J, Skuce RA, McBride SH, Menzies FD, McDowell SJW, Byrne AW, Handel IG, de Bronsvoort BMC. Bayesian latent class estimation of sensitivity and specificity parameters of diagnostic tests for bovine tuberculosis in chronically infected herds in Northern Ireland. Vet J. 2018;238:15–21. doi: 10.1016/j.tvjl.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Singhla T, Boonyayatra S, Chulakasian S, Lukkana M, Alvarez J, Sreevatsan S, Wells SJ. Determination of the sensitivity and specificity of bovine tuberculosis screening tests in dairy herds in Thailand using a Bayesian approach. BMC Vet Res. 2019;15:149. doi: 10.1186/s12917-019-1905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picasso-Risso C, Perez A, Gil A, Nunez A, Salaberry X, Suanes A, Alvarez J. Modelling the accuracy of two in-vitro bovine tuberculosis tests using a Bayesian approach. Front Vet Sci. 2019;6:261. doi: 10.3389/fvets.2019.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Álvarez J, Perez A, Bezos J, Marqués S, Grau A, Saez JL, Mínguez O, de Juan L, Domínguez L. Evaluation of the sensitivity and specificity of bovine tuberculosis diagnostic tests in naturally infected cattle herds using a Bayesian approach. Vet Microbiol. 2012;155:38–43. doi: 10.1016/j.vetmic.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Praud A, Boschiroli ML, Meyer L, Garin-Bastuji B, Dufour B. Assessment of the sensitivity of the gamma-interferon test and the single intradermal comparative cervical test for the diagnosis of bovine tuberculosis under field conditions. Epidemiol Infect. 2015;143:157–166. doi: 10.1017/S0950268814000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clegg TA, Duignan A, Whelan C, Gormley E, Good M, Clarke J, Toft N, More SJ. Using latent class analysis to estimate the test characteristics of the γ-interferon test, the single intradermal comparative tuberculin test and a multiplex immunoassay under Irish conditions. Vet Microbiol. 2011;151:68–76. doi: 10.1016/j.vetmic.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 12.de la Cruz ML, Branscum AJ, Nacar J, Pages E, Pozo P, Perez A, Grau A, Saez JL, de Juan L, Diaz R, Mínguez O, Alvarez J. Evaluation of the performance of the IDvet IFN-gamma test for diagnosis of bovine tuberculosis in Spain. Front Vet Sci. 2018;5:229. doi: 10.3389/fvets.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauzi S, Pasotto D, Amadori M, Archetti IL, Poli G, Bonizzi L. Evaluation of the specificity of the γ-interferon test in Italian bovine tuberculosis-free herds. Vet J. 2000;160:17–24. doi: 10.1053/tvjl.1999.0444. [DOI] [PubMed] [Google Scholar]
- 14.Antognoli MC, Remmenga MD, Bengtson SD, Clark HJ, Orloski KA, Gustafson LL, Scott A. Analysis of the diagnostic accuracy of the gamma interferon assay for detection of bovine tuberculosis in U.S. herds. Prev Vet Med. 2011;101:35–41. doi: 10.1016/j.prevetmed.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Cagiola M, Feliziani F, Severi G, Pasquali P, Rutili D. Analysis of possible factors affecting the specificity of the gamma interferon test in tuberculosis-free cattle herds. Clin Diagn Lab Immunol. 2004;11:952–956. doi: 10.1128/CDLI.11.5.952-956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghielmetti G, Landolt P, Friedel U, Morach M, Hartnack S, Stephan R, Schmitt S. Evaluation of three commercial interferon-γ assays in a bovine tuberculosis free population. Front Vet Sci. 2021;8:682466. doi: 10.3389/fvets.2021.682466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keck N, Boschiroli M-L, Smyej F, Vogler V, Moyen J-L, Desvaux S. Successful application of the gamma-interferon assay in a bovine tuberculosis eradication program: the French bullfighting herd experience. Front Vet Sci. 2018;5:27. doi: 10.3389/fvets.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gormley E, Doyle M, Duignan A, Good M, More SJ, Clegg TA. Identification of risk factors associated with disclosure of false positive bovine tuberculosis reactors using the gamma-interferon (IFNγ) assay. Vet Res. 2013;44:117. doi: 10.1186/1297-9716-44-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EFSA Panel on Animal Health and Welfare (AHAW) Scientific Opinion on the use of a gamma interferon test for the diagnosis of bovine tuberculosis. EFSA J. 2012;10:2975. doi: 10.2903/j.efsa.2012.2975. [DOI] [Google Scholar]
- 20.de Lisle GW, Green RS, Buddle BM. Factors affecting the gamma interferon test in the detection of bovine tuberculosis in cattle. J Vet Diagn Invest. 2017;29:198–202. doi: 10.1177/1040638716689114. [DOI] [PubMed] [Google Scholar]
- 21.Waters WR, Nonnecke BJ, Olsen SC, Palmer MV. Effects of pre-culture holding time and temperature on interferon-γ responses in whole blood cultures from Mycobacterium bovis-infected cattle. Vet Microbiol. 2007;119:277–282. doi: 10.1016/j.vetmic.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Faye S, Moyen JL, Gares H, Benet JJ, Garin-Bastuji B, Boschiroli ML. Determination of decisional cut-off values for the optimal diagnosis of bovine tuberculosis with a modified IFNγ assay (Bovigam®) in a low prevalence area in France. Vet Microbiol. 2011;151:60–67. doi: 10.1016/j.vetmic.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Praud A, Bourély C, Boschiroli ML, Dufour B. Assessment of the specificity of a gamma-interferon test performed with specific antigens to detect bovine tuberculosis, after non-negative results to intradermal tuberculin testing. Vet Rec Open. 2019;6:e000335. doi: 10.1136/vetreco-2019-000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waters WR, Thacker TC, Nonnecke BJ, Palmer MV, Schiller I, Oesch B, Vordermeier HM, Silva E, Estes DM. Evaluation of gamma interferon (IFN-γ)-induced protein 10 responses for detection of cattle infected with Mycobacterium bovis: comparisons to IFN-γ responses. Clin Vaccine Immunol. 2012;19:346–351. doi: 10.1128/CVI.05657-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiller I, Vordermeier HM, Waters WR, Whelan AO, Coad M, Gormley E, Buddle BM, Palmer MV, Thacker T, McNair J, Welsh M, Hewinson RG, Oesch B. Bovine tuberculosis: Effect of the tuberculin skin test on in vitro interferon gamma responses. Vet Immunol Immunopathol. 2010;136:1–11. doi: 10.1016/j.vetimm.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Estes DM, Closser NM, Allen GK. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-μ and mitogen. Cell Immunol. 1994;154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 27.Horii Y, Hirano T (1198) Pokeweed mitogen (PWM). In: Delves PJ (ed) Encyclopedia of immunology, 2nd edn. Elsevier, London, pp 1978–1879
- 28.R Core Team (2019) R: a language and environment for statistical computing
- 29.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiele C, Hirschfeld G. Cutpointr: Improved estimation and validation of optimal cutpoints in R. J Stat Softw. 2020;98:1–27. [Google Scholar]
- 31.Carstensen B, Plummer M, Laara E, Hills M (2021) Epi: a package for statistical analysis in epidemiology
- 32.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Meth. 2002;64:583–616. doi: 10.1111/1467-9868.00353. [DOI] [Google Scholar]
- 33.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBugs—a Bayesian modelling framework: concepts, structure and extensibility. Stat Comput. 2000;10:325–337. doi: 10.1023/A:1008929526011. [DOI] [Google Scholar]
- 34.Sturtz S, Ligges U, Gelman A. R2WinBUGS: a package for running WinBUGS from R. J Stat Softw. 2005;12:1–16. doi: 10.18637/jss.v012.i03. [DOI] [Google Scholar]
- 35.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7:457–511. doi: 10.1214/ss/1177011136. [DOI] [Google Scholar]
- 36.EFSA The European Union One Health 2019 Zoonoses Report. EFSA J. 2019;19:e06406. doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.More SJ, Radunz B, Glanville RJ. Lessons learned during the successful eradication of bovine tuberculosis from Australia. Vet Rec. 2015;177:224–232. doi: 10.1136/vr.103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodchild AV, Downs SH, Upton P, Wood JLN, de La Rua-Domenech R. Specificity of the comparative skin test for bovine tuberculosis in Great Britain. Vet Rec. 2015;177:258. doi: 10.1136/vr.102961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Hagan MJH, Ni H, Menzies FD, Pascual-Linaza AV, Georgaki A, Stegeman JA. Test characteristics of the tuberculin skin test and post-mortem examination for bovine tuberculosis diagnosis in cattle in Northern Ireland estimated by Bayesian latent class analysis with adjustments for covariates. Epidemiol Infect. 2019;147:e209. doi: 10.1017/S0950268819000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, Buddle BM, Thacker TC, Lyashchenko KP, Waters WR. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound Emerg Dis. 2010;57:205–220. doi: 10.1111/j.1865-1682.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- 41.de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res Vet Sci. 2006;81:190–210. doi: 10.1016/j.rvsc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Vordermeier M, Goodchild T, Clifton-Hadley R, de La Rua-Domenech R. The interferon-gamma field trial: Background, principles and progress. Vet Rec. 2004;155:37–38. [PubMed] [Google Scholar]
- 43.Sinclair JA, Dawson KL, Buddle BM. The effectiveness of parallel gamma-interferon testing in New Zealand’s bovine tuberculosis eradication programme. Prev Vet Med. 2016;127:94–99. doi: 10.1016/j.prevetmed.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Eirin ME, Macias A, Magnano G, Morsella C, Mendez L, Blanco FC, Bianco MV, Severina W, Alito A, Pando M, Singh M, Spallek R, Paolicchi FA, Bigi F, Cataldi AA. Identification and evaluation of new Mycobacterium bovis antigens in the in vitro interferon gamma release assay for bovine tuberculosis diagnosis. Tuberculosis. 2015;95:795–801. doi: 10.1016/j.tube.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Casal C, Bezos J, Díez-Guerrier A, Álvarez J, Romero B, de Juan L, Rodriguez-Campos S, Vordermeier M, Whelan A, Hewinson RG, Mateos A, Domínguez L, Aranaz A. Evaluation of two cocktails containing ESAT-6, CFP-10 and Rv-3615c in the intradermal test and the interferon-γ assay for diagnosis of bovine tuberculosis. Prev Vet Med. 2012;105:149–154. doi: 10.1016/j.prevetmed.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Schiller I, Waters WR, Vordermeier HM, Nonnecke B, Welsh M, Keck N, Whelan A, Sigafoose T, Stamm C, Palmer MV, Thacker T, Hardegger R, Marg-Haufe B, Raeber A, Oesch B. Optimization of a whole-blood gamma interferon assay for detection of Mycobacterium bovis-infected cattle. Clin Vaccine Immunol. 2009;16:1196–1202. doi: 10.1128/CVI.00150-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duignan A, Kenny K, Bakker D, Good M. Tuberculin PPD potency assays in naturally infected tuberculous cattle as a quality control measure in the Irish bovine tuberculosis eradication programme. Front Vet Sci. 2019;6:328. doi: 10.3389/fvets.2019.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Katani R, Schilling M, Kapur V. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis on dairy farms. Annu Rev Anim Biosci. 2016;4:155–176. doi: 10.1146/annurev-animal-021815-111304. [DOI] [PubMed] [Google Scholar]
- 49.Jungersen G, Huda A, Hansen JJ, Lind P. Interpretation of the gamma interferon test for diagnosis of subclinical paratuberculosis in cattle. Clin Diagn Lab Immunol. 2002;9:453–460. doi: 10.1128/CDLI.9.2.453-460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsen I, Boysen P, Kulberg S, Hope JC, Jungersen G, Storset AK. Bovine NK cells can produce gamma interferon in response to the secreted mycobacterial proteins ESAT-6 and MPP14 but not in response to MPB70. Infect Immun. 2005;73:5628–5635. doi: 10.1128/IAI.73.9.5628-5635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Álvarez J, de Juan L, Bezos J, Romero B, Sáez JL, Marqués S, Domínguez C, Mínguez O, Fernández-Mardomingo B, Mateos A, Domínguez L, Aranaz A. Effect of paratuberculosis on the diagnosis of bovine tuberculosis in a cattle herd with a mixed infection using interferon-gamma detection assay. Vet Microbiol. 2009;135:389–393. doi: 10.1016/j.vetmic.2008.09.060. [DOI] [PubMed] [Google Scholar]
- 52.Biet F, Boschiroli ML. Non-tuberculous mycobacterial infections of veterinary relevance. Res Vet Sci. 2014;97:S69–77. doi: 10.1016/j.rvsc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Roupie V, Alonso-Velasco E, van der Heyden S, Holbert S, Duytschaever L, Berthon P, Van Dosselaer I, Van Campe W, Mostin L, Biet F, Roles S, Huygen K, Fretin D. Evaluation of mycobacteria-specific gamma interferon and antibody responses before and after a single intradermal skin test in cattle naturally exposed to M. avium subsp. paratuberculosis and experimentally infected with M. bovis. Vet Immunol Immunopathol. 2018;196:35–47. doi: 10.1016/j.vetimm.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Buddle BM, Ryan TJ, Pollock JM, Andersen P, de Lisle GW. Use of ESAT-6 in the interferon-γ test for diagnosis of bovine tuberculosis following skin testing. Vet Microbiol. 2001;80:37–46. doi: 10.1016/S0378-1135(00)00375-8. [DOI] [PubMed] [Google Scholar]
- 55.Good M, Clegg TA, Costello E, More SJ. The comparative performance of the single intradermal test and the single intradermal comparative tuberculin test in Irish cattle, using tuberculin PPD combinations of differing potencies. Vet J. 2011;190:e60–e65. doi: 10.1016/j.tvjl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Bisschop PIH, Frankena K, Milne GM, Ford T, McCallan L, Young FJ, Byrne AW. Relationship between ambient temperature at sampling and the interferon gamma test result for bovine tuberculosis in cattle. Vet Microbiol. 2023;283:109778. doi: 10.1016/j.vetmic.2023.109778. [DOI] [PubMed] [Google Scholar]
- 57.Jenkins AO, Gormley E, Gcebe N, Fosgate GT, Conan A, Aagaard C, Michel AL, Rutten V. Cross reactive immune responses in cattle arising from exposure to Mycobacterium bovis and non-tuberculous mycobacteria. Prev Vet Med. 2018;152:16–22. doi: 10.1016/j.prevetmed.2018.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Impact of different cut-offs for Bovigam and IDvet in the sensitivity and specificity of the opposite test.
Additional file 2: Evolution of the animal-level specificity of the Bovigam and IDvet kit depending on the cut-off point (OD or S/P ratio) used to define positivity, depending on different covariables.
Additional file 3: Distribution of reactors depending on the age by region and production type.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.