Abstract
Fresh water sanitation and disinfection using a variety of chemical entities as chlorination agents is an essential public health intervention ensuring water safety for populations at a global scale. Recently, we have published our observation that the small molecule oxidant, innate immune factor, and chlorination agent HOCl antagonizes inflammation and photocarcinogenesis in murine skin exposed topically to environmentally relevant concentrations of HOCl. Chlorinated isocyanuric acid derivatives [including the chloramines trichloroisocyanuric acid (TCIC) and dichloroisocyanuric acid (DCIC)] are used worldwide as alternate chlorination agents serving as HOCl-precursor and stabilizer compounds ensuring sustained release in aqueous environments including public water systems, recreational pools, and residential hot tubs. Here, for the first time, we have examined the cutaneous TCIC-induced transcriptional stress response (in both an organotypic epidermal model and in AP-1 luciferase reporter SKH-1 mouse skin), also examining molecular consequences of subsequent treatment with solar ultraviolet (UV) radiation. Taken together, our findings indicate that cutaneous delivery of TCIC significantly enhances UV-induced inflammation (as profiled at the gene expression level), suggesting a heretofore unrecognized potential to exacerbate UV-induced functional and structural cutaneous changes. These observations deserve further molecular investigations in the context of TCIC-based freshwater disinfection with health implications for populations worldwide.
INTRODUCTION
Fresh water sanitation and disinfection using a variety of chemical entities as chlorination agents is an essential public health intervention ensuring water safety for populations at a global scale (as recognized by the United Nations’ sustainable development goals) (1, 2). The small molecule electrophile, oxidant, and chlorination agent hypochlorous acid (HOCl), apart from its multifaceted biological role as a crucial antimicrobial factor of the innate immune system, is a key mediator of chlorination stress targeting microbes as relevant to chemical disinfection ensuring safety of drinking water and recreational water use (2–11). Remarkably, biomedical and toxicological impact of chemical exposure-induced chlorination stress targeting human epithelial (cutaneous, gastrointestinal, and respiratory) tissue remain largely undefined, representing an unexplored area of the skin exposome and redoxosome (2, 11, 12).
Serving an important endogenous microbicidal and immune-modulatory role in the context of the innate immune system, HOCl has recently emerged as an important mediator of numerous human pathologies including tissue response to trauma and wound healing, vascular endothelial damage, respiratory illness, neurodegeneration, cancer, and senescence (13–17). Regulatory guidelines stipulate acceptable HOCl environmental exposure levels [≤ 5 ppm (equaling ≤ 100 μM) following US (CDC/EPA) guidelines] relevant to cutaneous impact as it occurs during recreational freshwater use, for example in the context of public pool sanitation (5, 7, 10). Only a limited number of studies have addressed the potential health impact associated with water chlorination as it occurs for drinking water disinfection and recreational use, focusing mostly on (i) HOCl (in equilibrium with OCl−) as the ultimate chlorination agent and (ii) HOCl-precursor chemicals (designed to stabilize and release the reactive intermediate). Moreover, chlorination byproducts (CBPs including chloroform) are known to be associated with numerous irritant, organotoxic, and potentially carcinogenic effects impacting human health as a function of environmental exposure (documented for example in bladder cancer, liver damage, disinfectant-related asthma, and allergic contact dermatitis) (2, 3, 5, 18–22).
Chlorinated isocyanuric acid derivatives [including the chloramines trichloroisocyanuric acid (TCIC; CAS#: 87-90-1), dichloroisocyanuric acid (DCIC), and isocyanuric acid chlorinated in situ in the presence of HOCl] are used worldwide as alternate chlorination agents serving as HOCl-precursor and stabilizer compounds that ensure sustained release in aqueous environments such as drinking water reservoirs and swimming pools used according to EPA-recommended hazard assessment and concentration levels (≤ 300 μM) (23–26). Ubiquitous use of these agents is predicated on various seemingly favorable chemical features including synthetic accessibility, shelf life, established safety profile, and photostability, a property of special significance in the context of pool disinfection where the well-established UV-instability of HOCl/OCl− compromises sustained maintenance of antimicrobial chlorination levels (23–28). Remarkably, biological effects of TCIC on epithelial human tissues are largely understudied, and most available evidence has been obtained performing standard regulatory toxicity assessment (including acute irritant exposure and long-term feeding models). Indeed, the role of TCIC-associated chlorination stress in skin deterioration, particularly in the context of solar UV co-exposure, remains unexplored even though human exposure is ubiquitous, and pharmacokinetics of human exposure to isocyanuric acid and its chlorinated derivates (ingested orally by recreational swimmers) have been described (23).
Acute and chronic skin photodamage is a well-established consequence of human exposure to solar ultraviolet (UV) radiation, involved in photoaging and photocarcinogenesis, and environmental modulation (enhancement or attenuation) of skin photodamage impacted by additional factors of the skin exposome (such as arsenic and polyaromatic hydrocarbons) is well documented (29–38). Interestingly, with reference to co-exposure scenarios involving environmental disinfectants and solar UV (such as in the context of recreational swimming pool use), only limited experimental data are available documenting cutaneous pathology and toxicological effects downstream of this environmentally relevant co-exposure (3, 5). Recently, we have published our findings employing co-exposure models indicating that topical HOCl suppresses inflammatory photocarcinogenesis in SKH-1 high risk mouse skin (2, 11). In accordance with this observation, a topical HOCl regimen suppressed acute UV-induced inflammation and AP-1-controlled transcriptional activity in transgenic SKH-1 luciferase-reporter mouse skin as revealed by oxidative stress RT2 Profiler™ array analysis, substantiating the established anti-inflammatory effect of topical HOCl harnessed clinically for therapeutic intervention targeting inflammatory pathologies including pruritus, atopic and radiation dermatitis, and psoriasis (2, 14).
Here, for the first time, skin effects of topical TCIC-exposure were examined first in an organotypic model of human epidermis and then in AP-1 luciferase reporter SKH-1 mouse skin examining the molecular interaction that occurs upon subsequent treatment with solar UV, an experimental approach mimicking environmental co-exposure. These novel data demonstrate that topical TCIC significantly enhances UV-induced inflammation, suggesting a heretofore unrecognized potential to exacerbate UV-induced functional and structural cutaneous changes. This co-exposure might be relevant to specific environmental exposure scenarios, impacting for instance human skin exposed to topical TCIC (pool disinfectant) and UV (solar radiation). Given the ubiquitous nature of human TCIC exposure in the context of freshwater disinfection, implications for skin health (as investigated here for the first time) deserve further molecular investigations.
MATERIALS AND METHODS
All methods (- including human skin cell culture, cell viability analysis by flow cytometry, caspase 3 activation assay, detection of intracellular oxidative stress, human epidermal reconstructs, epidermal AP-1 luciferase mouse model, comparative RT2 Profiler™ gene expression array analysis, single RT-qPCR expression analysis, and tissue immunohistochemistry -) were performed according to our previously published procedures as specified in detail in ‘Supporting Materials’ (11, 39–46).
Statistical analysis:
Data sets were analyzed employing analysis of variance (ANOVA) with Tukey’s posthoc test using the GraphPad Prism 9.1.0 software (Prism Software Corp., Irvine, CA); in respective bar graphs (analyzing more than two groups), means without a common letter differ (p < 0.05) as published before (11). Experiments involved at least three independent individual replicates per data point. Nonparametric data analysis of murine experimentation (bioluminescence assessment) was performed using the Mann–Whitney test. Differences between groups were considered significant at p < 0.05.
RESULTS AND DISCUSSION
The freshwater disinfectant TCIC induces cytotoxicity in human HaCaT keratinocytes characterized by annexin V/PI-positivity, cleavage of procaspase 3, and induction of redox dysregulation
Using cultured HaCaT human keratinocytes, cytotoxicity was first evaluated by flow cytometric analysis revealing the dose response relationship of TCIC-induced cell death (≤ 500 μM in regular medium; 24 h continuous exposure; Fig. 1a), observable at high micromolar concentrations (≥ 250 μM). Subsequently, time course analysis indicated loss of viability observable already within 60 min TCIC exposure (500 μM) causing approximately 35% reduction in viability with cells staining positive exclusively for annexin V (Fig. 1b). The proportion of dead cells increased as a function of exposure time, with more than 95% reduction in viability observable at 24 h. At that time point, all cells displayed positivity for annexin V, with substantial co-staining for propidium iodide (detectable in approximately 50% of dying cells) indicative of late-stage apoptotic cell death, an observation supported by detection of TCIC-induced cleavage of procaspase 3 (Fig. 1c). We then examined the induction of oxidative stress in response to treatment with TCIC employing flow cytometric analysis of dye oxidation [assessing conversion of the fluorogenic precursor 2′,7′-dihydrodichlorofluorescein-diacetate (DCFH-DA); Fig. 1d] (41). Indeed, oxidative dye conversion with formation of dichlorofluorescein was observable within 1h exposure time (TCIC: 250 μM). In contrast, at low TCIC concentrations (≤ 100 μM), ROS formation was not detectable.
Figure 1. Exposure to the freshwater disinfectant TCIC induces cell death with caspase 3 activation and oxidative stress in human HaCaT keratinocytes.
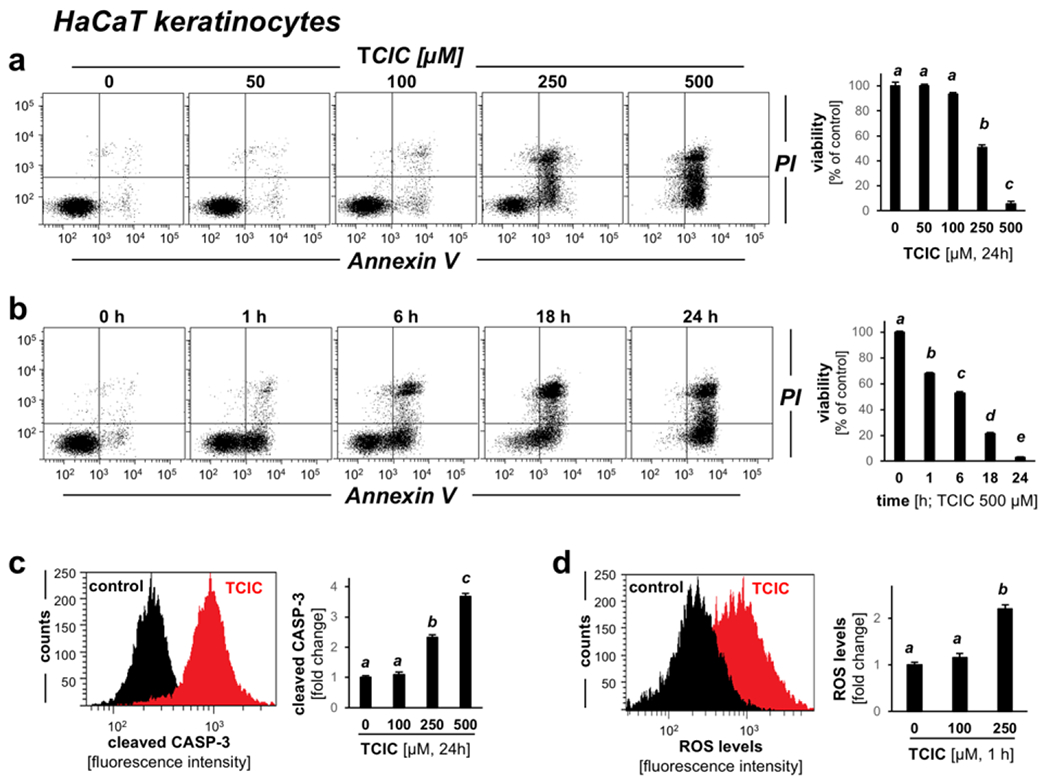
(a) Flow cytometric analysis of TCIC effects (0-500 μM; in culture medium, 24 h) on cell viability. (b) Time course analysis (500 μM; in cell culture medium). (c) Flow cytometric analysis of TCIC-induced (≤ 250 μM μM, 24 h) procaspase-3 cleavage (histogram: control versus 250 μM). (d) Redox dysregulation elicited by TCIC exposure (≤ 250 μM; 1 h) as assessed by 2’,7’-dihydrodichlorofluorescein-diacetate (DCFH-DA) oxidation (histogram: control versus 250 μM).
Array analysis reveals induction of redox stress response gene expression in human reconstructed epidermis (EpiDerm™) exposed to topical TCIC
Next, after elucidating acute toxicity towards cultured HaCaT keratinocytes, a human organotypic epidermal model was exposed to TCIC (100 μM, PBS, 6 h) with subsequent gene expression analysis employing the Oxidative Stress Plus RT2 Profiler™ PCR Array technology (TCIC versus PBS only; Fig. 2) (11). As a result of TCIC exposure, expression of the following genes was associated with statistically significant changes (≥ three-fold) elicited by TCIC exposure as analyzed by scatter plot depiction (Fig. 2a), volcano plot depiction [expression differential versus respective p-value (Fig. 2b)], and quantitative overview summarizing gene expression changes [fold change ≥ 2; p value ≤ 0.05 (Fig. 2c)]: MB (encoding myoglobin; 14.4-fold), BNIP3 (encoding BCL2 interacting protein 3; 7.1-fold), TXNRD1 (encoding thioredoxin reductase 1; 3.3-fold), and GCLM (encoding glutamate -cysteine ligase, modifier subunit; 3.3-fold); in contrast, TCIC-induced downregulation by at least three-fold was observed with GSR [encoding glutathione reductase; 3.5-fold].
Figure 2. Redox gene expression analysis reveals transcriptional changes induced by the freshwater disinfectant TCIC in an organotypic model of human epidermis.
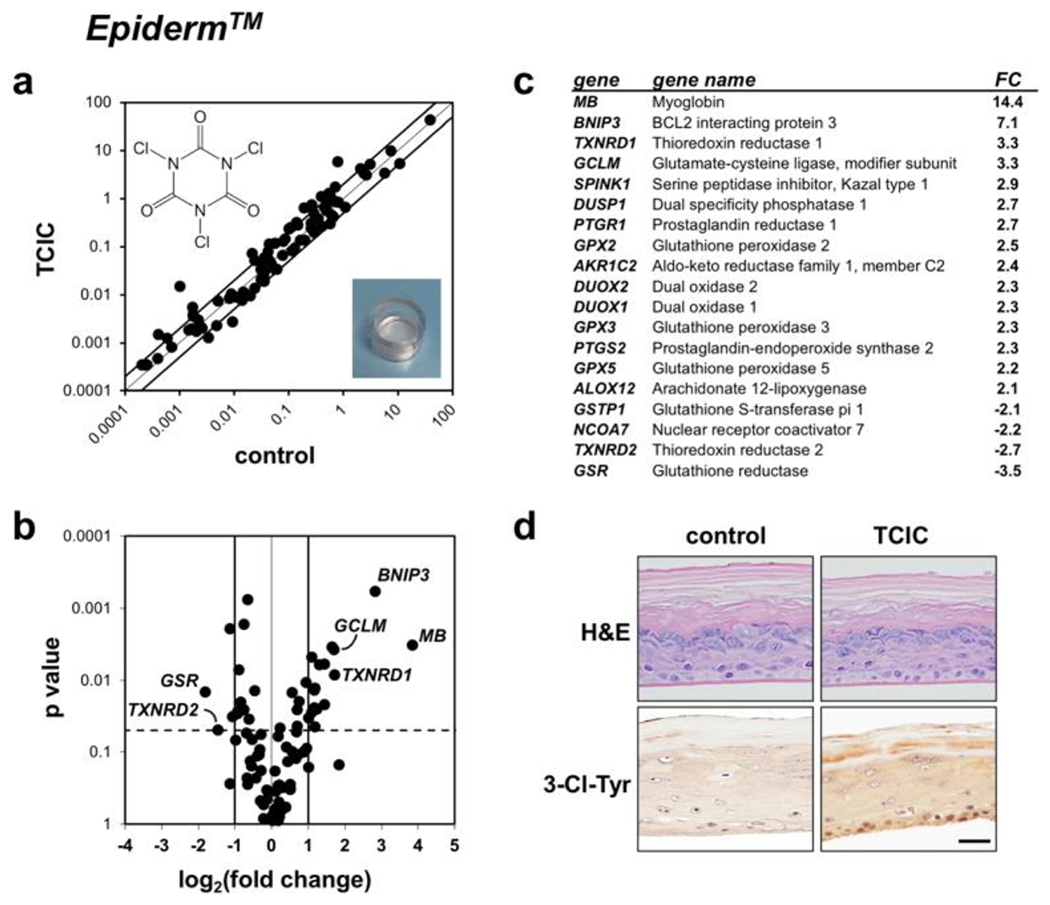
(a-c) EpiDerm™ was exposed topically to TCIC (100 μM, 6 h; chemical structure as displayed) in PBS (w/v), followed by RT2 Profiler™ PCR Array gene expression analysis; panel a: scatter plot; panel b: volcano plot; panel c: quantitative summary; cut-off line: fold change ≥ 2; p <0.05]. (d) IHC analysis of representative EpiDerm™ specimens exposed to control or TCIC treatment examining H&E staining (top) and 3-Cl-Tyr (bottom); graphic scale: 50 μm.
Subsequent IHC analysis confirmed tissue integrity (H&E stain; Fig. 2d) and TCIC-induced introduction of 3-chloro-tyrosine-epitopes (3-Cl-Tyr; Fig. 2d; bottom panels), indicative of posttranslational modification as a result of chlorination observed before in chlorination stress-associated pathologies including UV-damage, inflammatory dysregulation, metabolic syndrome, septic shock, malignancy, and cellular senescence (11, 14–17).
In summary, topical cutaneous TCIC exposure [performed at a concentration relevant to environmentally-exposed human skin (100 μM)] induces a oxidative stress-related transcriptional response detectable in a human organotypic epidermal model (Epiderm™). It is interesting to note that examination of HOCl-induced transcriptional responses at the mRNA level (as assessed by us before using the same Epiderm™ exposure model) has already profiled an HOCl-induced oxidative epidermal stress response; however, our data indicate that identity of specific genes (responsive to chlorination agents) differs as a function of the specific chlorination-inducing chemical entity (i.e., HOCl versus the chloramine TCIC) that determines reactivity and pharmacokinetic parameters of skin delivery, an unexplored topic to be examined in adequate detail by future experimentation (11).
Topical TCIC enhances AP-1 transcriptional activity in response to acute UV exposure in a murine cutaneous exposure model
Recently, we have shown that transgenic SKH-1 reporter mice engineered for luciferase expression under the control of activator protein 1 (AP-1) are a suitable model allowing (i) the detection of UV-induced AP-1 transcriptional activation and (ii) screening of topical chemopreventive agents that antagonize this UV-sensitive inflammatory pathway (11, 44, 45). We therefore examined the impact of cutaneous TCIC pre-exposure on AP-1 signaling elicited by subsequent UV exposure, mimicking co-exposure as it would occur in human swimmer’s skin exposed to solar UV and pool-associated disinfectants. To this end, murine cutaneous exposure was performed employing TCIC (100 μM in carrier), generating four treatment groups: (i) ‘TCIC’, (ii) ‘carrier’, (iii) ‘TCIC plus UV’ (UVB: 275 mJ/cm2), or (iv) ‘carrier plus UV’ (Fig. 3a). Employing this in vivo exposure model, it was observed that UV treatment caused AP-1 activation as indicated by upregulation of luciferase-dependent luminescence intensity (by more than 500-fold over carrier control) (Fig. 3b), a finding consistent with our previous data using this model exploring HOCl-effects on skin inflammation after UV exposure (11). Strikingly, TCIC used as a single agent, did not induce a cutaneous luminescence signal; however, if applied in combination with UV, TCIC pre-treatment (preceding UV exposure) potentiated UV-induced luminescence intensity by more than 100% (consistent with AP-1 hyperactivation), an observation indicative of the inflammation-enhancing effects associated with this environmentally-relevant chloramine.
Figure 3. Topical TCIC exacerbates transcriptional activity in murine AP-1 reporter skin.
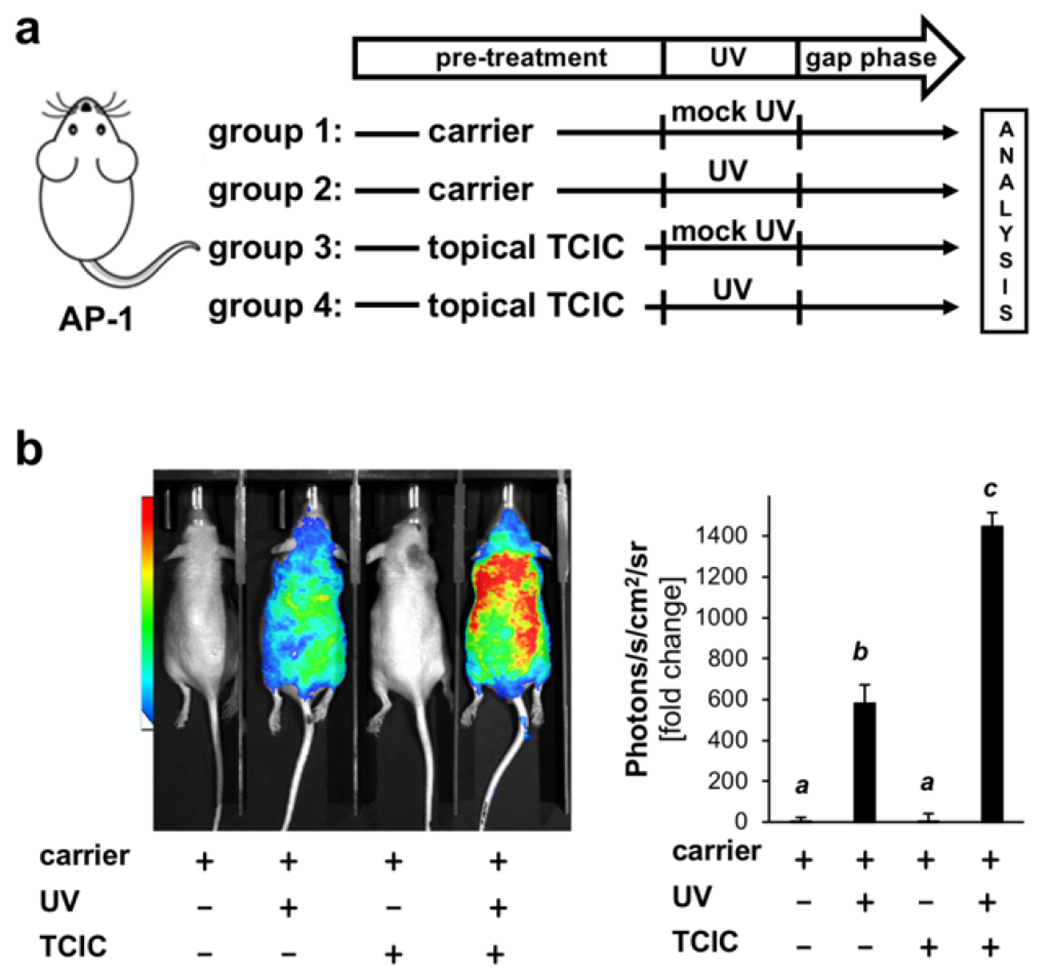
(a) Treatment scheme: Mice were pre-exposed using ‘TCIC (100 μM) in carrier’ or ‘carrier only’ followed by UVB (275 mJ/cm2) and bioluminescence determination 24 h after; in addition, control groups were included (‘carrier’ versus ‘UV exposure’ only; ‘groups 1–4’, n ≥ 3). Timeline of TCIC-treatment: Carrier (with or without TCIC) was administered twice (dorsal skin; 24 h and 1 h pre-UV). (b) Quantitative determination of bioluminescent signal in vivo.
Array analysis indicates TCIC-potentiation of UV-induced oxidative stress and inflammatory gene expression detectable in SKH-1 murine skin
Following our expression analysis performed in a TCIC-exposed organotypic epidermal model (Fig. 2), we examined redox and inflammatory gene expression responses at the mRNA level comparing solar UV-induced gene expression changes in SKH-1 mouse skin subjected to ‘TCIC’ or ‘carrier only’ pretreatment (Fig. 4).
Figure 4. Gene expression array analysis identifies changes in murine skin characteristic of topical co-exposure to TCIC and UV.
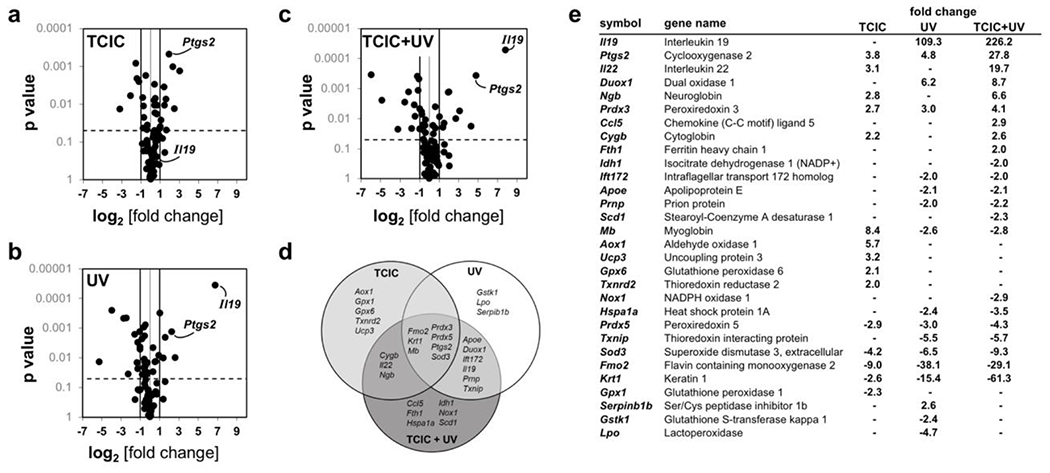
Oxidative Stress RT2 Profiler™ PCR gene expression: (a) TCIC versus untreated control; (b) UV versus untreated control; (c) TCIC + UV versus untreated control; (d) Venn diagram displaying ‘TCIC’ versus ‘UV’ versus ‘TCIC + UV’ (all relative to untreated control). (e) TCIC/UV-induced transcriptional response as a function of topical treatment regimen.
TCIC-induced gene expression changes (Oxidative Stress RT2 Profiler™ PCR Array; greater than three-fold) were detectable as follows (upregulated expression): Mb (encoding myoglobin; 8.4-fold), Aox1 (encoding aldehyde oxidase 1; 5.7-fold), Ptgs2 (encoding cyclooxygenase 2; 3.8-fold), Ucp3 (encoding uncoupling protein 3; 3.2 fold), and Il22 (encoding interleukin 22; 3.1 fold); downregulated expression: Fmo2 (encoding flavin containing monooxygenase 2; 9.0 fold) and Sod3 (encoding superoxide dismutase 3, extracellular; 4.2 fold; Fig. 4a and e).
Next, UV-induced gene expression in murine skin was profiled, characterized by pronounced changes at the mRNA level (greater than three-fold); upregulated expression: Il19 [encoding interleukin 19; 109.3-fold], Duox1 [encoding dual oxidase 1; 6.2-fold], Ptgs2 (encoding cyclooxygenase 2; 4.8-fold), and Prdx3 (encoding peroxiredoxin 3; 3.0-fold); downregulated expression: Fmo2 (encoding flavin containing monooxygenase 2; 38.1 fold), Krt1 (encoding keratin 1; 15.4 fold), Sod3 (encoding superoxide dismutase 3, extracellular; 6.5 fold), Txnip (encoding thioredoxin interacting protein; 5.5 fold), Lpo (encoding lactoperoxidase; 4.7 fold), and Prdx5 (encoding peroxiredoxin 5; 3.0 fold; Fig. 4b and e).
Strikingly, if TCIC pretreatment was followed by UV-exposure (Fig. 4c and e), exacerbated gene expression changes were observed as follows: UV exposure (following TCIC) upregulated expression of Il19, Duox1, and Ptgs2 (Figs. 3b and 4c&e). Likewise, enhancement of UV-induced downregulation of gene expression by TCIC pretreatment was observed with Krt1 and Sod3. Differential gene expression as a function of treatment (TCIC/UV) is summarized by Venn diagram analysis revealing identity of genes exclusively sensitive to single agent exposure (TCIC or UV) or combination treatment (Fig. 4d). Remarkably, expression of Mb, an emerging molecular factor involved in progression of epithelial tumors (including oxidative stress and hypoxia response), acting beyond its well-established role in muscle cell oxygen- and iron-binding, was upregulated in response to TCIC exposure (if applied as single agent) and downregulated upon single agent (UV only) or co-exposure (TCIC/UV), an effect to be explored by follow up experimentation (47, 48). In the future, additional investigations will comprehensively address the TCIC-associated cutaneous impact resulting from topical exposure focusing on the importance of residence time, concentration, pH, and pre-/ co-/ post-exposure regimens. It remains to be seen, how effects of TCIC that differ from HOCl are related to its chloramine entity that might dictate differential biological outcomes. Likewise, the specific mechanistic involvement of AP-1 (and other UV-responsive stress response transcription factors as NFκB) underlying TCIC-modulation of UV responses awaits further clarification. In this context it should be mentioned that HOCl-induced posttranslational modification of redox-sensitive cysteine residues is known to cause IκB kinase (IKK) oxidation, thereby attenuating subsequent NFκB-dependent inflammatory gene expression with downregulation of major mediators including PTGS2 (2, 11, 14, 17). Likewise, numerous other redox sensitive signaling pathways have been shown to be HOCl- and chloramine-responsive (including p53, p38 MAPK, and Keap1/NRF2 as reviewed recently), to be examined by future investigations comparing HOCl versus TCIC effects (2).
Taken together, our observations, obtained for the first time in a relevant in vivo exposure model (SKH-1 mouse skin exposed to topical TCIC followed by UV radiation), indicate that acute topical TCIC induces transcriptional changes in skin, potentiating UV-induced oxidative stress- and inflammation-related gene expression if used as a combinatorial agent in SKH-1 mouse skin. These prototype data will expanded exploring (i) impact of carrier (Vanicream™ as compared to other more relevant exposure vehicles), (ii) TCIC dose response, (iii) specific impact of UV spectral coverage (UVA versus UVB versus combined full spectrum solar simulated UV) and exposure regimen (e.g. topical treatment with TCIC that occurs pre-, versus co-, versus post-UV delivery), and (iv) other factors that determine translational relevance of these prototype findings.
RT-qPCR- and IHC-based tissue analyses confirm TCIC enhancement of molecular changes observable in murine skin exposed to acute UV
Next, employing additional RT-qPCR probes [not contained on the expression array (Fig. 4)], we further characterized TCIC-modulation of transcriptional changes detectable at the mRNA level in response to UV exposure (Fig. 5). TCIC-dependent transcriptional changes (measured versus UV only) were observable with the following genes encoding established inflammatory mediators including Il1b (encoding interleukin 1 beta; 7.4 fold), Il6 (encoding interleukin 6; 4.0 fold), Il10 (encoding interleukin 10; 3.0 fold), Tnf (encoding tumor necrosis factor; 2.0 fold), while expression of other genes interrogated by our focused inflammatory expression panel (Cxcl15, Il17a, Il33) did not display any responsiveness to either UV, TCIC, or the combination thereof (data not shown). Using our independent single RT-qPCR approach, we also confirmed the TCIC-dependent upregulation of UV-responsive inflammatory genes already examined in array format [Ptgs2 (5.0 fold), Il19 (2.4 fold); Fig. 5, bottom panels].
Figure 5. RT-qPCR analysis confirms that topical TCIC enhances UV-induced inflammatory transcriptional responses detectable at the mRNA level in mouse skin.
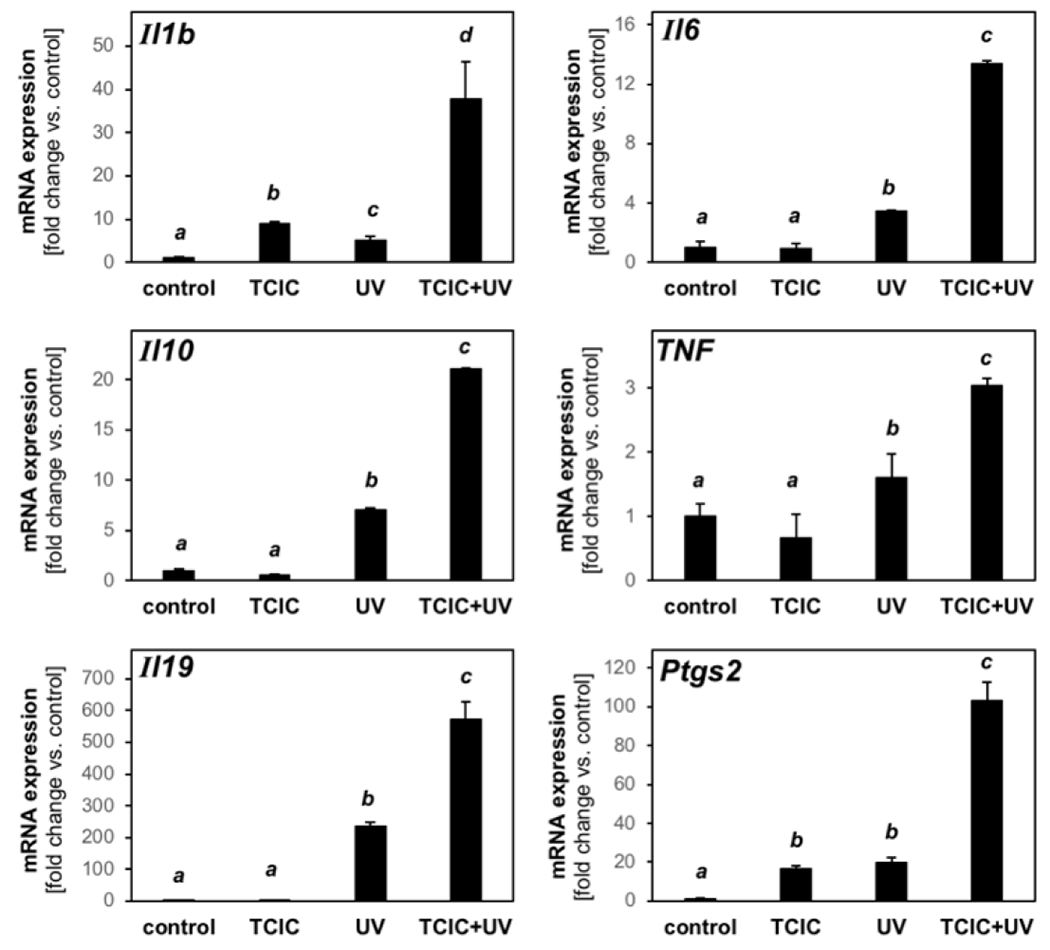
After mRNA preparation from dorsal mouse skin, single RT-qPCR assessment of inflammatory gene expression (Ptgs2, Tnf, Il1b, Il6, Il10, Il19) was performed (control: untreated (‘carrier only’).
Next, following our observation obtained at the transcriptional level (Figs. 4 and 5), IHC skin analysis indicated enhanced detectability of IL-19 and COX-2 that occurred as a function of TCIC/UV cotreatment (Fig. 6). Interestingly, COX-2 tissue staining was responsive to TCIC or UV single agent treatment, an effect further enhanced by cotreatment. Furthermore, 3-chloro-tyrosine epitopes were detectable in all samples exposed to TCIC, a finding reminiscent of our observation in epidermal reconstructs (Fig. 2) and earlier research on HOCl (11). Likewise, epidermal expression of the proliferation marker PCNA was elevated in response to UV (but not TCIC used as a single agent), an effect enhanced by TCIC co-exposure. Accordingly, hyperplastic changes characteristic of actinic insult (as assessed by epidermal thickness) were enhanced by approximately 30% as a result of TCIC/UV co-exposure as compared to treatment with UV only (Fig. 6; top panels).
Figure 6. IHC analysis confirms that topical TCIC enhances expression of inflammatory stress response genes in murine skin.
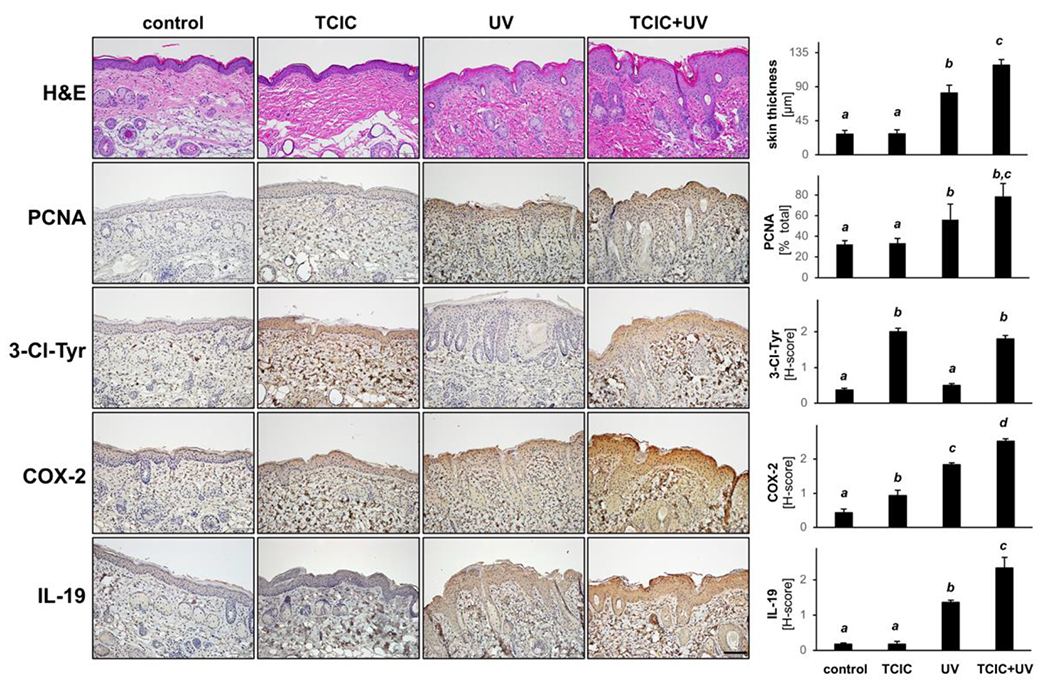
FFPE-skin sections (thickness: 5 μm) were analyzed by IHC (hematoxylin/eosin, 3-Cl-Tyr, COX-2, IL-19, PCNA; scale bar: 100 μm).
CONCLUSION
Cellular chlorination stress as a result of environmental exposure is an important molecular factor impacting public health, both with regard to recreational (i.e. swimming pool) and drinking water use, representing a topic of essential importance to global freshwater use and safety in the 21st century (2). In the context of ubiquitous epithelial (cutaneous, pulmonary, gastrointestinal, etc.) exposure, insufficient experimental evidence exists that examines toxicity (as a result of single agent exposure or in combination with solar UV) of HOCl or HOCl-precursors such as isocyanuric acid-derived chloramines (e.g. TCIC etc.) used globally for freshwater disinfection. Our data, obtained in relevant human epidermal organotypic and murine reporter mouse models, indicate that the ubiquitous chloramine and HOCl precursor TCIC is a potent inducer of epithelial chlorination stress, enhancing cutaneous UV-induced inflammatory gene expression in vivo. Strikingly, TCIC-induced modulation of UV-effects on skin is opposed to those observed with topical application of HOCl applied in similar carrier and dose regimens, i.e. HOCl-dependent suppression versus TCIC-dependent enhancement of UV-responses (11). Thus, the differential effects of topical TCIC exposure (versus HOCl) on UV-induced cutaneous responses (likely attributable to the chloramine nature and delayed HOCl-release by this chemical entity dictating distinct chemical reactivity and cutaneous pharmacokinetics) deserve further mechanistic exploration in relevant exposure models. Future translational examinations will address the molecular basis, applicability to human exposure scenarios, and potential relevance to public health as suggested by our TCIC-directed explorations examining cutaneous impact in combination with UV exposure in vivo for the first time.
Supplementary Material
Funding:
Supported in part by grants from the National Institutes of Health (1R01CA229418, 1R03CA230949, 1R21ES029579, 1P01CA229112, ES007091, ES006694, and UA Cancer Center Support Grant CA023074). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors state no conflict of interest.
REFERENCES
- 1.Moe CL, Rheingans RD (2006) Global challenges in water, sanitation and health, J Water Health, 4 Suppl 1, 41–57. [PubMed] [Google Scholar]
- 2.Snell JA, Jandova J, Wondrak GT (2022) Hypochlorous Acid: From Innate Immune Factor and Environmental Toxicant to Chemopreventive Agent Targeting Solar UV-Induced Skin Cancer, Front Oncol, 12, 887220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Oragnization (2006) Guidelines for safe recreational water environments: VOLUME 2 SWIMMING POOLS AND SIMILAR ENVIRONMENTS, ISBN 92 4 1546808. [Google Scholar]
- 4.T.H.I.C.P.A.C. (HICPAC) (2008) Guideline for Disinfection and Sterilization in Healthcare Facilities, Centers for Disease Control and Prevention (CDC). [Google Scholar]
- 5.Richardson SD, DeMarini DM, Kogevinas M, Fernandez P, Marco E, Lourencetti C, Balleste C, Heederik D, Meliefste K, McKague AB, Marcos R, Font-Ribera L, Grimalt JO, Villanueva CM (2010) What’s in the pool? A comprehensive identification of disinfection by-products and assessment of mutagenicity of chlorinated and brominated swimming pool water, Environ Health Perspect, 118, 1523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prest EI, Hammes F, van Loosdrecht MC, Vrouwenvelder JS (2016) Biological Stability of Drinking Water: Controlling Factors, Methods, and Challenges, Front Microbiol, 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Environmental Protection Agency (EPA) (2018) Ground water and drinking water - chlorination https://www.epa.gov/ground-water-and-drinking-water/emergency-disinfection-drinking-water(accessed: 5-29-2022).
- 8.Giarratana N, Rajan B, Kamala K, Mendenhall M, Reiner G (2021) A sprayable Acid-Oxidizing solution containing hypochlorous acid (AOS2020) efficiently and safely inactivates SARS-Cov-2: a new potential solution for upper respiratory tract hygiene, Eur Arch Otorhinolaryngol, 278, 3099–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kampf G, Todt D, Pfaender S, Steinmann E (2020) Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents, J Hosp Infect, 104, 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EPA (US Environmental Protection Agency); List N. Disinfectants for use against SARS-CoV-2. [Google Scholar]
- 11.Jandova J, Snell J, Hua A, Dickinson S, Fimbres J, Wondrak GT (2021) Topical hypochlorous acid (HOCl) blocks inflammatory gene expression and tumorigenic progression in UV-exposed SKH-1 high risk mouse skin, Redox Biol, 45, 102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schalka S, Silva MS, Lopes LF, de Freitas LM, Baptista MS (2022) The skin redoxome, J Eur Acad Dermatol Venereol, 36, 181–95. [DOI] [PubMed] [Google Scholar]
- 13.Casciaro M, Di Salvo E, Pace E, Ventura-Spagnolo E, Navarra M, Gangemi S (2017) Chlorinative stress in age-related diseases: a literature review, Immun Ageing, 14, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung TH, Zhang LF, Wang J, Ning S, Knox SJ, Kim SK (2013) Topical hypochlorite ameliorates NF-kappaB-mediated skin diseases in mice, J Clin Invest, 123, 5361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer G (2018) HOCl and the control of oncogenesis, J Inorg Biochem, 179, 10–23. [DOI] [PubMed] [Google Scholar]
- 16.Ulfig A, Leichert LI (2021) The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens, Cell Mol Life Sci, 78, 385–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu TW, Gammon ST, Yang P, Fuentes D, Piwnica-Worms D (2021) Myeloid cell-derived HOCl is a paracrine effector that trans-inhibits IKK/NF-kappaB in melanoma cells and limits early tumor progression, Sci Signal, 14, eaax5971. [DOI] [PubMed] [Google Scholar]
- 18.Helenius I, Haahtela T (2000) Allergy and asthma in elite summer sport athletes, J Allergy Clin Immunol, 106, 444–52. [DOI] [PubMed] [Google Scholar]
- 19.Schoefer Y, Zutavern A, Brockow I, Schafer T, Kramer U, Schaaf B, Herbarth O, von Berg A, Wichmann HE, Heinrich J, L.s. group (2008) Health risks of early swimming pool attendance, Int J Hyg Environ Health, 211, 367–73. [DOI] [PubMed] [Google Scholar]
- 20.Font-Ribera L, Kogevinas M, Zock JP, Nieuwenhuijsen MJ, Heederik D, Villanueva CM (2009) Swimming pool attendance and risk of asthma and allergic symptoms in children, Eur Respir J, 34, 1304–10. [DOI] [PubMed] [Google Scholar]
- 21.Goma A, de Lluis R, Roca-Ferrer J, Lafuente J, Picado C (2017) Respiratory, ocular and skin health in recreational and competitive swimmers: Beneficial effect of a new method to reduce chlorine oxidant derivatives, Environ Res, 152, 315–21. [DOI] [PubMed] [Google Scholar]
- 22.Pardo A, Nevo K, Vigiser D, Lazarov A (2007) The effect of physical and chemical properties of swimming pool water and its close environment on the development of contact dermatitis in hydrotherapists, Am J Ind Med, 50, 122–6. [DOI] [PubMed] [Google Scholar]
- 23.Suppes LM, Abrell L, Dufour AP, Reynolds KA (2014) Assessment of swimmer behaviors on pool water ingestion, J Water Health, 12, 269–79. [DOI] [PubMed] [Google Scholar]
- 24.Batsalova T, Kolchakova D, Dzhambazov B (2018) In vitro cytotoxicity of cyanuric acid and selected derivatives, Toxicol Forensic Med Open J 3, 14–21. [Google Scholar]
- 25.Wahman DG (2018) Chlorinated Cyanurates: Review of Water Chemistry and Associated Drinking Water Implications, J Am Water Works Assoc, 110, E1–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang YH, Shi HJ (2022) UV/chlorinated cyanurates as an emerging advanced oxidation process for drinking water and potable reuse treatments, Water Res, 211, 118075. [DOI] [PubMed] [Google Scholar]
- 27.Tilstam U, Weinmann H (2002) Trichloroisocyanuric acid: A Safe and Efficient Oxidant, Org Process Res Dev, 6, 384–93. [Google Scholar]
- 28.Sancier KM, Brady AP, Lee WW (1964) Absorption spectra of solutions of cyanuric acid and its chlorinated derivatives, Spectrochimica Acta, 20, 397–403. [Google Scholar]
- 29.Cadet J, Douki T (2018) Formation of UV-induced DNA damage contributing to skin cancer development, Photochem Photobiol Sci, 17, 1816–41. [DOI] [PubMed] [Google Scholar]
- 30.Wondrak GT (2007) Let the sun shine in: mechanisms and potential for therapeutics in skin photodamage, Curr Opin Investig Drugs, 8, 390–400. [PubMed] [Google Scholar]
- 31.Wondrak GT (2019) Sunscreen-Based Skin Protection Against Solar Insult: Molecular Mechanisms and Opportunities., Fundamentals of Cancer Prevention, ed Alberts DaH LM (Springer Science & Business Media; ), 377–404. [Google Scholar]
- 32.Wang Y, Gao D, Atencio DP, Perez E, Saladi R, Moore J, Guevara D, Rosenstein BS, Lebwohl M, Wei H (2005) Combined subcarcinogenic benzo[a]pyrene and UVA synergistically caused high tumor incidence and mutations in H-ras gene, but not p53, in SKH-1 hairless mouse skin, Int J Cancer, 116, 193–9. [DOI] [PubMed] [Google Scholar]
- 33.Boffetta P, Nyberg F (2003) Contribution of environmental factors to cancer risk, Br Med Bull, 68, 71–94. [DOI] [PubMed] [Google Scholar]
- 34.Nair S, Kekatpure VD, Judson BL, Rifkind AB, Granstein RD, Boyle JO, Subbaramaiah K, Guttenplan JB, Dannenberg AJ (2009) UVR exposure sensitizes keratinocytes to DNA adduct formation, Cancer Prev Res (Phila), 2, 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein CB, Leszczynska J, Hickey C, Rossman TG (2007) Further evidence against a direct genotoxic mode of action for arsenic-induced cancer, Toxicol Appl Pharmacol, 222, 289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saladi R, Austin L, Gao D, Lu Y, Phelps R, Lebwohl M, Wei H (2003) The combination of benzo[a]pyrene and ultraviolet A causes an in vivo time-related accumulation of DNA damage in mouse skin, Photochem Photobiol, 77, 413–9. [DOI] [PubMed] [Google Scholar]
- 37.Baudouin C, Charveron M, Tarroux R, Gall Y (2002) Environmental pollutants and skin cancer, Cell Biol Toxicol, 18, 341–8. [DOI] [PubMed] [Google Scholar]
- 38.Cope RB, Imsilp K, Morrow CK, Hartman J, Schaeffer DJ, Hansen LG (2003) Exposure to soil contaminated with an environmental PCB/PCDD/PCDF mixture modulates ultraviolet radiation-induced non-melanoma skin carcinogenesis in the Crl:SKH1-hrBR hairless mouse, Cancer Lett, 191, 145–54. [DOI] [PubMed] [Google Scholar]
- 39.Williams JD, Bermudez Y, Park SL, Stratton SP, Uchida K, Hurst CA, Wondrak GT (2014) Malondialdehyde-derived epitopes in human skin result from acute exposure to solar UV and occur in nonmelanoma skin cancer tissue, J Photochem Photobiol B, 132, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perer J, Jandova J, Fimbres J, Jennings EQ, Galligan JJ, Hua A, Wondrak GT (2020) The sunless tanning agent dihydroxyacetone induces stress response gene expression and signaling in cultured human keratinocytes and reconstructed epidermis, Redox Biol, 36, 101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Justiniano R, de Faria Lopes L, Perer J, Hua A, Park SL, Jandova J, Baptista MS, Wondrak GT (2021) The Endogenous Tryptophan-derived Photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) is a Nanomolar Photosensitizer that Can be Harnessed for the Photodynamic Elimination of Skin Cancer Cells in Vitro and in Vivo, Photochem Photobiol, 97, 180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SL, Justiniano R, Williams JD, Cabello CM, Qiao S, Wondrak GT (2015) The Tryptophan-Derived Endogenous Aryl Hydrocarbon Receptor Ligand 6-Formylindolo[3,2-b]Carbazole Is a Nanomolar UVA Photosensitizer in Epidermal Keratinocytes, J Invest Dermatol, 135, 1649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jandova J, Park SL, Corenblum MJ, Madhavan L, Snell JA, Rounds L, Wondrak GT (2022) Mefloquine induces ER stress and apoptosis in BRAFi-resistant A375-BRAF(V600E) /NRAS(Q61K) malignant melanoma cells targeting intracranial tumors in a bioluminescent murine model, Mol Carcinog, 61, 603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickinson SE, Melton TF, Olson ER, Zhang J, Saboda K, Bowden GT (2009) Inhibition of activator protein-1 by sulforaphane involves interaction with cysteine in the cFos DNA-binding domain: implications for chemoprevention of UVB-induced skin cancer, Cancer Res, 69, 7103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blohm-Mangone K, Burkett NB, Tahsin S, Myrdal PB, Aodah A, Ho B, Janda J, McComas M, Saboda K, Roe DJ, Dong Z, Bode AM, Petricoin EF 3rd, Calvert VS, Curiel-Lewandrowski C, Alberts DS, Wondrak GT, Dickinson SE (2018) Pharmacological TLR4 Antagonism Using Topical Resatorvid Blocks Solar UV-Induced Skin Tumorigenesis in SKH-1 Mice, Cancer Prev Res (Phila), 11, 265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bair WB 3rd, Cabello CM, Uchida K, Bause AS, Wondrak GT (2010) GLO1 overexpression in human malignant melanoma, Melanoma Res, 20, 85–9 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flonta SE, Arena S, Pisacane A, Michieli P, Bardelli S (2009) Expression and functional regulationof myoglobin in epithelial cancers, Am J Pathol, 175, 201–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galluzzo M, Pennacchietti S, Rosano S, Comoglio PM, Michieli P (2009) Prevention of hypoxia by myoglobin expression in human tumor cells promotes differentiation and inhibits metastasis, J Clin Invest 119, 865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


