Abstract
The placenta is both the literal and metaphorical black box of pregnancy. Measurement of the function of the placenta has the potential to enhance our understanding of this enigmatic organ and serve to support obstetric decision making. Advanced imaging techniques are key to support these measurements. This review summarises emerging imaging technology being used to measure the function of the placenta and new developments in the computational analysis of these data. We address three important examples where functional imaging is supporting our understanding of these conditions: fetal growth restriction, placenta accreta, and twin-twin transfusion syndrome.
Introduction: from biology to the clinic
The placenta is perhaps the most interesting multifunctional animal organ to have ever evolved. The placenta acts as a critical exchange organ for the baby in utero, mediating transfer of nutrients and oxygen between mother and fetus whilst keeping their two circulations entirely separate. Placenta-like organs are found across nearly all branches of animals, from primates to sharks, with variable degrees of complexity in anatomy and exchange efficiency. 1,2 However, the wide variability of placental appearance and structure between species make the human placenta anatomically distinct from many common laboratory and agricultural animal models, 3 meaning that directly studying the human placenta is critical. In vivo imaging of the placenta in human pregnancy is thus a valuable tool for understanding normal and abnormal placental function.
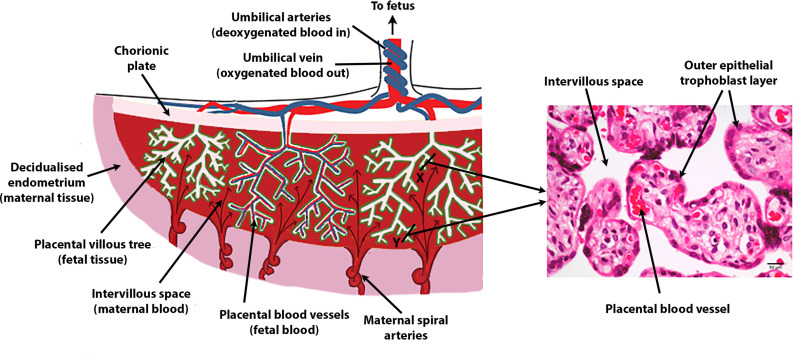
The human placenta is disc-shaped and weighs around 500g at term. Indeed, ‘placenta’ is the latin term for ‘flat cake’, and the size and shape are not dissimilar. Structurally, what initially appears as a relatively solid disc is in fact comprised of extensively and densely branched villous trees, which branch from the chorionic plate (the aspect of the placenta closest to the fetus). 4 These villous trees are grouped into discrete amorphous functional units termed lobules (or occasionally cotyledons) of which 20–25 are ordinarily visible in the human placenta. For most of pregnancy, maternal blood flows from the uterine circulation and circulates around the outside of these villous trees. Exchange then occurs across the outer epithelial layer of the placenta, which has a surface area of 12 m2 by the end of pregnancy. 4,5 Within the placental lobules, a complex-branched fetal vascular network carries deoxygenated blood from the fetus to the exchange surface with the mother, and returns oxygenated blood back to the fetus via the umbilical vein (Figure 1).
Figure 1.
Illustrations of human placental features and cellular relationships.
Over the course of pregnancy, the placenta extensively remodels the maternal uterine vasculature, to facilitate a 15-fold increase in blood flow to the placenta. 6 This increase in nutrient and oxygen supply is crucial for adequate fetal growth. To achieve this, specialised placental cells (called trophoblasts) migrate out from the placenta and invade the endometrium, the inner layer of the maternal uterine cavity and beyond, up to the level of the uterine muscle or myometrium. Once in this tissue the trophoblast cells invade into glands, veins, and lymph vessels of the uterine wall to provide nutritional support of the embryo with substances in maternal plasma and uterine gland secretion products. The trophoblast cells replace the muscle in the walls of the terminal spiral arteries and remodel them eventually into wide non-vasoactive tubes that open out to supply blood to the placenta. 7 Hormones and other paracrine factors secreted by the placenta act to remodel the larger upstream vessels, which double in size by mid-gestation. 8 Together, these changes enable a higher volume of blood to be delivered to the placenta, but at an appropriate velocity of flow around the placental villous tree to optimise exchange efficiency.
The successful growth and development of the placenta, and its adaptation of the maternal uterine circulation are critical for pregnancy success. However, inadequacies in multiple points across this system can occur, resulting in pregnancy disorders such as pre-eclampsia (when inflammatory signals from the placenta cause dangerously high maternal blood pressure), or fetal growth restriction (FGR, when the fetal growth rate decelerates or stagnates in utero) due to placental insufficiency. 9–11 Conversely, abnormal placental attachment can occur at the site of scars from previous caesarean section or other uterine incisions or trauma. This condition termed placenta accreta spectrum can lead to life-threatening maternal haemorrhage at the time of delivery, as well as the need for hysterectomy to completely remove the invasive placenta. 12 Identical twins and higher multiple pregnancies also create unique scenarios in utero due to vascular connections within the shared placenta, potentially resulting in discrepancies in blood distribution between the fetuses called twin-to-twin transfusion syndrome (TTTS) that can be life-threatening for both if not treated in utero. 13 As a result, the placenta is a clinically important target for developments in imaging technology, so that scientists and clinicians can examine the structure and function of the organ in utero and improve maternal and neonatal outcomes.
This review highlights some common imaging technologies used in pregnancy, with a focus on new developments to quantify placental function from imaging.
What is being used at the moment to measure placenta function?
Ultrasound
Ultrasound is the most routinely used clinical imaging tool for investigation of the placenta in vivo. Obstetric ultrasound typically assesses fetal growth through serial fetal measurements and placental shape, cord insertion and uterine position using gray scale. Pregnancies commonly have placental blood flow inputs and outputs monitored via assessment of uterine, umbilical, fetal middle cerebral arterial and ductus venosus Doppler ultrasound flow waveforms (Figure 2). Doppler ultrasound provides a functional interpretation of the blood flow in these vessels, inferring information on placental perfusion and fetal wellbeing by the shape, direction and magnitude of their flow velocity. In chronic hypoxia, the fetal circulation is redistributed towards the brain away from other circulatory beds, especially to the fetal kidneys and lower limbs leading to increased middle cerebral artery perfusion.
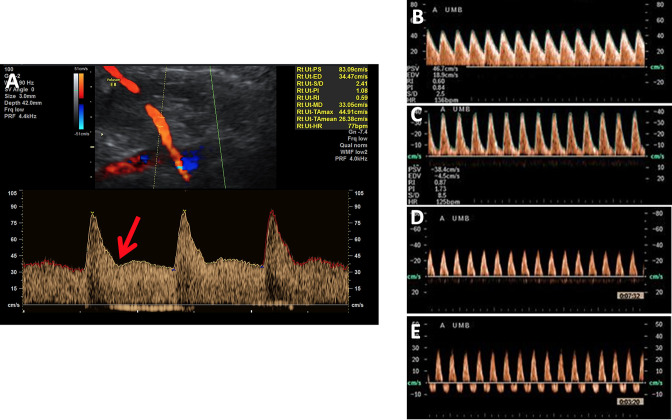
Figure 2.
a: Prediastolic notching of the uterine artery waveform (red arrow) and raised pulsatility index (PI) at 22 weeks indicates placental insufficiency and has a high positive predictive value for fetal growth restriction b: Normal umbilical artery Doppler Increasing resistance in the umbilical artery leads to the development of raised PI (c), absent (d) and then reversed end diastolic flow (e).
Maternal placental perfusion can be estimated in vivo with measurement of uterine artery volume blood flow, pulsatility and resistance indices. The uterine arteries vasodilate from early in normal pregnancy, providing increased volume blood flow to the uterus and developing fetus. Doppler analysis of the uterine arteries from the first trimester can indicate a poorly developed utero-placental circulation, leading to placental insufficiency and fetal growth restriction (FGR). Typical sonographic findings include an increased pulsatility index and pre-diastolic notching in the uterine artery Doppler waveform indicating high vascular resistance. There is a well-established association between increased uterine artery pulsatility index in the late second trimester, and the development of early onset pre-eclampsia and FGR,. 14–16 However, the sensitivity and specificity are poor. 17
The fetoplacental circulation can be examined in vivo with umbilical artery Doppler measurements and is used clinically to give an indication of fetal wellbeing. 18 An increased umbilical artery pulsatility index and reduced or reversed end-diastolic flow indicates increasing resistance in the fetoplacental vascular bed, as seen in placental insufficiency. 19,20 This relationship has been validated in animal models, where progressive embolisation of fetal vessels in sheep resulted in progression from normal to absent to reversed end diastolic flow in the umbilical artery. 21
Redistribution of the fetal cardiac output in response to developing fetal hypoxia can also be detected antenatally, via a reduction in the middle cerebral artery (MCA) pulsatility index indicating cerebrovascular dilatation 22,23
While not yet used clinically at this stage, more detailed Doppler analyses using power or colour Doppler are beginning to be used to produce detailed maps of the circulation at the utero-placental interface 24,25 and in the feto-placental circulation. 26 These emerging ultrasound techniques interpret the ultrasound scatter due to the presence of red blood cells to provide maps representing blood flow velocity (colour Doppler) or speed (power Doppler). They may provide more detailed functional interpretation than their predecessors, but care must be taken to ensure that machine settings are comparable when interpreting these data, 27 and so ensuring consistency and reproducibility in methodologies, and significant validation is required before routine clinical use. As a result, clinical ultrasound relies heavily on morphometric rather than functional imaging whilst analysis of Doppler waveforms can be susceptibility to intrasubject variability and the use of compound measurements involving ratios.
MRI
MRI is safe in pregnancy 28,29 and the whole placenta may be imaged at any gestational age. Although many new techniques have been attempted, the use of MRI in the assessment of placental function in the clinic is not widespread. The reasons for this are complex, but the promise of MRI is to provide quantitative measurement of placental function. At this stage, many MR techniques have been proposed, but very few, if any, have been validated with a causal mechanism that supports the correlations observed. 30 Like emerging ultrasound technologies, this validation is critical for clinical adoption, and this absence of physiologically grounded knowledge is restricting placental MRI from further integration in maternal-fetal medicine.
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is an imaging technique that enables spatial and quantitative characterisation of the maternal perfusion in normal or pathological conditions such as placental insufficiency with an injection of contrast agent. 31–34 DCE-MRI is performed by using fast imaging sequences (e.g., gradient echo) that are repeatedly applied over the organ of interest during bolus administration of a contrast agent. The temporal uptake of contrast agent measured from the image time-series provides quantitative information about tissue blood perfusion. One of the first studies in which gadolinium contrast was used to assess human placental function in second and third trimester was performed by Marcos et al.. 35 The diagnostic potential of DCE-MRI has also been assessed in placenta accreta spectrum (PAS) disorders (placenta previa and abnormal invasive placenta). 32 In this study DCE-MRI allowed the extraction of tissue enhancement parameters of the uterus and materno-placental circulation that differed significantly between pregnancies with PAS and normal pregnancies. DCE-MRI has also been used in animal models. 36–39 Previous studies in mice have shown differences in placental perfusion between normal and disease conditions at a given gestation. 40–43 More recent studies have showed potential in detecting abnormal flow patterns in placentas affected by fetal growth restriction whilst ruling out PAS. 44 Despite relatively slow trans-placental transfer, DCE-MRI clinically is limited by the use of exogenous contrast agents based on gadolinium which have an unknown safety profile in pregnancy. Contrast agents may cross to the fetus and are recirculated in the amniotic fluid and questions remain about long term accumulation of gadolinium. 28 As a result, it is only recommended for clinical use if it significantly enhances diagnostic performance and is expected to improve fetal or maternal outcome such as in the case of PAS. 45 This motivates the development of non-contrast agent-based techniques such as diffusion imaging to measure placental function.
An alternative to DCE-MRI for measuring the properties of the maternal circulation is Arterial Spin Labelled MRI. This technique makes use of magnetic pre-labelling of blood as it passes into the field of view. As a result, this technique is free of exogenous contrast agents. Blood flow is measured by subtracting labelled and un-labelled images and as a result this technique is susceptible to motion artefacts between images which can corrupt the result. Additionally, the relatively low SNR requires high field strength and multiple averages to be acquired. Despite this, the technique has potential in the placenta to reveal the properties especially of the materno-placental circulation 46–49 and when combined with sophisticated image analysis techniques, the extracted parameters have the potential to be quite robust. 50,51
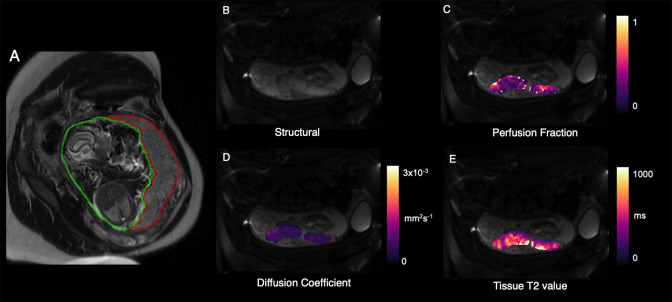
Diffusion-weighted (DW) MRI uses the random motion of water molecules within tissue as contrast, providing information on diffusion within placental tissue and the exchange properties of the maternal and fetal circulations. 52 In DW-MRI, perfusion can be approximated using intravoxel incoherent motion (IVIM) model. 53,54 In IVIM-DW imaging, perfusion is evaluated by exploiting the fact that blood in each voxel has pseudorandom translational motion within the capillaries, providing access to perfusion when using small magnetic field gradients during the MR pulse sequences. Placental diffusion and perfusion changes measured with IVIM-MRI technique have been gaining recognition 55–57 (Figure 3). A recent study demonstrated the effect of maternal sleep position on utero-placental and feto-placental blood flow oxygenation in healthy late gestation pregnancy using DW-MRI. 58 Diffusion-tensor (DT) MRI, an extension of DW-MRI, computes diffusion anisotropy. Fractional anisotropy could be detected by DT-MRI to differentiate functional placental tissue. 59
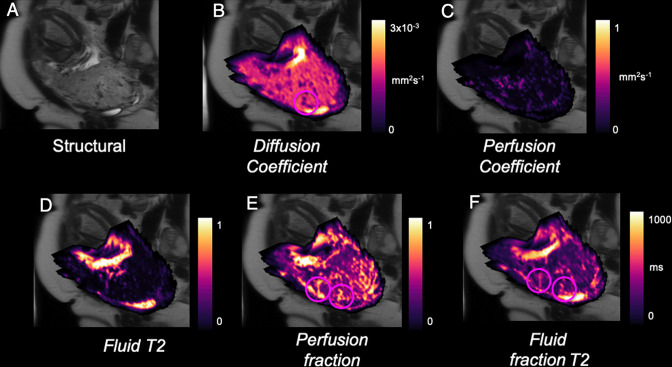
Figure 3.
Example parametric maps of placental function from MRI in an example of PAS. a) T2-weighted structural image, b) apparent diffusivity of placenta c) Pseudo-diffusivity of placenta[53,68] d) Placental T2[69], e) Placental perfusion and free-fluid signal [52]. Circles highlight vascular features close to the placental-myometrial border.
The sensitivity of T2* to oxygenation and the rapidity of acquiring a T2*-weighted gradient echo image mean that oxygen flux can be monitored dynamically. This technology was originally developed in the context of investigating functional changes in perfusion and oxygen usage in the human brain in fMRI. 60–65 However, it also has important applications in pregnancy and has been shown to be sensitive to changing placental oxygen levels during uterine contractions and external changes to the maternal oxygen level. 66,67
Although MRI technology shows promise detecting placental abnormalities, it has not been adopted yet in clinical practice likely due to several challenges in accessibility. Both examination costs and comfort play a role in the relatively limited availability of studies on MRI of human placental function and, despite the rich range of contrasts available, both MR structural and temporal resolution is comparably low compared to ultrasound. If these challenges can be overcome, MRI could be a valuable method to detect abnormal placental function in clinical practice.
What clinical questions could measurement of placental function answer?
The shape and structure of the feto-placental vasculature has been linked to a number of pregnancy pathologies. Changes are seen throughout the vasculature ranging from the largest chorionic blood vessels that are considered the framework upon which the entire placental vascular tree is built, down to the smallest capillary blood vessels. 68–71 This disrupted vasculature is evident in pre-eclampsia, FGR, 72–75 diabetic pregnancies, 76 placenta accreta 77–79 , preterm premature rupture of membranes and identical twin pregnancies. 80–82 Measurements of the vascular topology and function will help us to model how the placenta is functioning in these critical pathologies.
Application to Fetal Growth Restriction (FGR)
Abnormal placental function leads to FGR and maternal hypertensive disease. Preventing FGR or its early identification reduces the risk of stillbirth and has the potential to improve lifelong health. Ultimately poor placental function results in sustained nutrient restriction and fetal hypoxia, which can lead to long-term neurocognitive and cardiovascular impairment in the child and adult. 83,84 But even low birthweight (<2.5 kg) is associated with dramatically increased risks of cardiovascular and metabolic disease in later life. 85,86 Antenatal identification of abnormal placental function prior to development of fetal and maternal sequelae is therefore a health priority, but developing technologies to achieve this has proved challenging. International advances in placental imaging now provide the realistic prospect of detecting abnormal function early enough in gestation to allow treatments to be administered to those at risk. Novel treatments are currently being developed with the potential to treat placental insufficiency, so developing better ways to precisely measure changes in placental function is a priority.
Clinical care is currently guided by assessment of maternal risk factors, measurement of uterine artery blood flow using Doppler, monitoring fetal growth and fetal heart rate variability using antenatal cardiotocography (CTG). Maternal circulating proteins such as the growth factors PAPP-A and PlGF 87,88 are insufficiently sensitive and specific to predict placental insufficiency and FGR alone, and small fetal size is not equivalent to fetal growth restriction; fetuses whose estimated fetal weight is within the normal range, but that have placental insufficiency with reduced growth velocity can easily be missed. These gaps in our knowledge mean that care in these pregnancies may not be optimal. The ability of MRI to detect differences in the placentas of pregnancies with early onset FGR (<32 weeks of gestation) associated with placental insufficiency is well established. 75,89,90 As yet unknown is the ability of imaging to measure placental insufficiency more broadly in circumstances where anatomical differences in feto-placental circulation are more subtle, but the effects of resulting chronic hypoxia may still be clinically important although less easily detected.
There are no known risks to the fetus from MRI, including within the first trimester, 28,29 and the whole placenta may be imaged at any gestational age, something that is not possible to achieve using ultrasound in the second half of pregnancy. Whilst also falling with gestational age, 91,92 T2 and T2* relaxation time, which relate to the structure and oxygen level of the tissue, decrease substantially in placental insufficiency compared to normal placentas. 66,93 The impedance of diffusion of water molecules, and thus oxygen, is increased in placental insufficiency compared to normal placentas 94,95 and the vascular perfusion fraction and oxygenation, measured using diffusion imaging with placenta-specific modelling, is reduced in placental insufficiency compared to normal placentas. 75,96–98
Late stillbirth is fortunately uncommon (3.9 per 1,000 UK births) but is more frequently found in the situation of undetected late onset FGR where the fetus may be only slightly growth restricted. It is now well recognized that maternal supine sleep position in late pregnancy is independently associated with an increased risk of stillbirth, 99 and all females are recommended to go to sleep on their side-in the third trimester of pregnancy. The pathological mechanism leading to stillbirth is thought to be the gravid uterus compressing the inferior vena cava (IVC) when a female lies in the supine position during late pregnancy. 100 Using MRI, it has recently been shown that compared to the left lateral position, maternal supine position in healthy late pregnancy is associated with reduced utero-placental blood flow and oxygen transfer across the placenta. There was an average 6.2% reduction in oxygen delivery to the fetus and an average 11% reduction in fetal umbilical venous blood flow. 58 Thus even in healthy late gestation pregnancy, maternal position significantly affects oxygen transfer across the placenta and may partly explain late stillbirth in vulnerable fetuses.
After these findings, the next steps are 1) to validate physiological markers from MRI by making use of pre-clinical studies and 2) to broaden clinical studies to broader phenotypes of placental insufficiency such as late onset FGR (>32 weeks of gestation), and females with reduced fetal movements. This will allow the assessment of less severe, but more complex phenotypes where current clinical tools to assess fetal wellbeing such as doppler ultrasound and CTG are limited and further allow a link to be established between MR parameters and early changes in placental function in human pregnancy and generate evidence-based hypotheses about the defined pathological changes in the placenta observed in humans. Future work in this area will improve our understanding of placental pathophysiology and advance the usefulness of imaging in caring for females and their babies in pregnancy.
Application to placenta accreta
Placenta Accreta Spectrum (PAS) Disorders involve abnormal placental adherence and vascular disruption to the myometrium, leading to life-threatening maternal haemorrhage. 101,102 The condition has a prevalence of 0.04–0.42% pregnancies. 103 However, the incidence of PAS rises with successive caesarean section deliveries, with a rate of 4.1% in females with one prior caesarean delivery and 13.3% in females with two or more previous caesareans. 104 As the rate of caesarean section has reached>50% in some countries, the optimal diagnosis and management of PAS pregnancy is becoming critical in obstetrics. 105
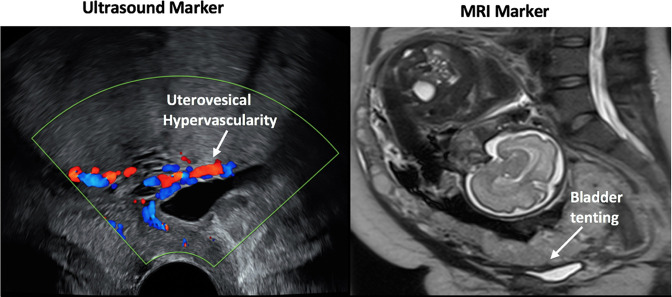
The placenta is separated from the myometrium by the decidua. 106 Injury to the endometrium, through uterine surgery such as caesarean section, can result in a decidual deficit in subsequent pregnancies causing abnormal placentation whereby chorionic villi directly abut the myometrium and extra villous trophoblast invasion is increased. 107,108 A histopathological specimen of a placenta accreta case is shown in Figure 4. Failure to recognise and manage PAS disorders at delivery can lead to massive post-partum haemorrhage with a mortality rate of 2.6–7% as the placenta fails to detach from the uterine wall and surrounding tissues. 109 It is important to correctly diagnose this disorder, with accurate assessment of the extent of abnormal attachment for better surgical planning. This is currently being performed by subjective interpretation of typical sonographic markers using 2D grey-scale and Doppler imaging, with MRI only used as an adjunct. 101,110 A standardised protocol for ultrasound reporting of findings (e.g., abnormal placental lacunae, or myometrial thinning) has been published by the European Working Group on Abnormally Invasive Placenta (EW-AIP). 111,112 These findings have a varying degree of sensitivity and specificity. 110 Similarly, some structural MRI signs include dark T2 intraplacental bands, placental heterogenous signal intensity, uterine bulging, focal interruption of the myometrium and tenting of the bladder. 108,110 An example of these radiological signs is illustrated in Figure 5. These morphologic markers are highly subjective, even when functional MRI methods such as DCE, DWI, and IVIM are employed 32 and have varying sensitivity and specificity. Recently standard imaging protocols, reporting terminology and structured reports have been proposed. This should lead to improved reporting and comparison between different studies and techniques.Figure 6 113–115
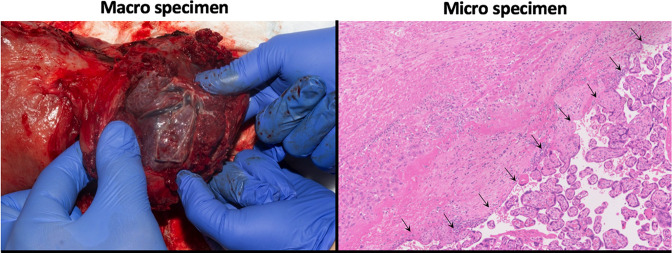
Figure 4.
Macro (left) and Micro (right) specimens for a PAS disorder patient. Arrows show: Chorionic villi directly related to the myometrium with no intervening decidua. Appearances are in keeping with placenta accreta.
Figure 5.
Examples of morphological ultrasound (left) and MRI (right) markers in a patient with PAS disorder confirmed on histopathology of the caesarean hysterectomy specimen. The imaging signs are labelled within the figure.
Figure 6.
Example parametric maps of placental function from MRI in an example of TTTS. a) T2 weighted structural image and whole placenta segmentation (red), b) Structural image with no diffusion weighting, c) Placental perfusion 52 ,d) apparent diffusivity of placenta and e) Tissue T2 value 93
The limitation of these methods includes low spatial resolution and difficulty assessing vascular invasion. 32,116,117 Combined T2 relaxometry (T2R) and intravoxel incoherent motion (IVIM) signal models can be used to separate signals from fetal and maternal blood pools over a region of interest of previous scar tissue and suspected abnormal placentation. 52,90,118 By studying how parameters from this multicompartmental model vary in PAS disorders it may be possible to identify differences in vascularity and perfusion for an objective quantification of abnormal placentation and to measure the effects of abnormal trophoblast invasion. Similarly, advances in image reconstruction technology 119,120 applied to areas of abnormal attachment, may better distinguish between degrees of abnormality and thus support clinical decisions for delivery.
Application to Twin-twin transfusion syndrome
Around one-third of twin pregnancies in the UK share a placenta. The mortality rate associated with these twins is 11%, with 44% of this mortality caused by a condition known as Twin-to-Twin transfusion syndrome (TTTS), which results from an interfetal transfusion imbalance because of placental chorionic vascular anastomoses. 121 TTTS manifests as fluid overload in the recipient twin and fluid depletion in the donor. 122 Untreated TTTS is associated with the death of one or more fetuses in more than 80% of pregnancies 123,124 and is accompanied by high rates of morbidity in surviving fetuses. 125 Current treatment via fetoscopic laser ablation (FLA) of selected chorionic vessels is supported by level one randomised control trial data. 126 A modification to the technique known as the Solomon technique, involves coagulation of the vascular equator of the chorionic surface after selective FLA. This has now been widely accepted as being more effective, but damages a larger proportion of the shared placenta. 127 A further condition called Twin Anaemia Polycythaemia Sequence (TAPS) can also develop due to a discordance in haemoglobin levels. This may also be treatable using FLA. 128
Ultrasound plays a vital role in the management of multiple pregnancies with a shared placenta starting with a correct determination of the chorionicity in the first trimester to ensure more intensive surveillance of high risk pregnancies. 129 In the following weeks ultrasound remains crucial in the detection of complicated fetal outcomes from 16 weeks onwards in view of potential fetal therapy. 130–132 Although ultrasound helps in the detection of TTTS, 133 it remains impossible to measure placental blood sharing accurately in these pregnancies and therefore the progression of the severity of TTTS is currently unpredictable. Careful follow-up is therefore required because of the higher chance of sudden and significant intertwin transfusion imbalances after 26 weeks in a twin pregnancy with growth discordance. 134 Furthermore, although TTTS occurs mainly before 26 weeks, it can occur at any time during a continuing twin pregnancy. 135
Improving outcomes relies on more detailed pre-surgical planning, predicting the effect of different formations of connecting vessels and modelling changes in deep placental function as these changes are made. Advanced MRI methods have the potential to extract combined information relevant to blood redistribution in TTTS. 46,52,69,136 In the future, MRI models could provide a mapping of vessel anastomoses and vascular density to establish correspondence between in vivo appearance and function and to make computational predictions based on pre-surgical MRI. Such a computational model would allow to predict the intraoperative effects of laser vessel ablation on the flow dynamics of twin placentae during intervention.
Several core imaging technologies have been developed recently that have potential for enhancing surgery in TTTS. For instance, advanced feto-placental super-resolution reconstruction and segmentation 120,137–139 may be adapted to allow visualization and measurement of the placental chorionic vascular tree. 70,140,141 This is essential information prior to laser ablation of the vascular anastmomoses. Furthermore, advances allow measurement of placental structure and function using MRI 52,142,143 ; and recent techniques for blood flow simulation allow placental chorionic vessel blood flow modelling. 141,144 Combined these techniques could provide prediction of the haemodynamic response during surgery.
Post-delivery, the morphology of the placenta can be assessed and compared with outcome using several protocols. 70,140,145 High-resolution microCT data enable a high-resolution reconstruction of the placental vascular structure for computational flow modelling without affecting subsequent placental histology. 145 This modelling may allow the prediction of how conditions such as TTTS and TAPS can develop from certain configurations of placental anastomoses.
Looking forward: Mixed models and computational modelling
Combining data from MRI
The power of recent MRI models arises from the ability to combine information from different sources of contrast. Several attempts have been made to use combinations of MRI sequences to disentangle information about placental perfusion and oxygenation. 146–148
Imaging from MRI typically is used to represent a single parameter of interest such as T2 or diffusivity. 91,93,149 By acquiring acquisitions across multiple contrasts, it becomes possible to isolate the contribution to the T2 or diffusivity from separate placental sources, 147,148,150 for instance to measure properties of the maternal and fetal circulations separately. This approach has been used in FGR 92 and to analyses the effect of maternal position on placental function. 151 Attempts have also been made to carry out this separation using ultrasound. 152 Current interest in uterine contractions and the effect on placental function suggests that the combination of information from dynamic BOLD MRI could be combined with markers of volume or shape change in the placenta. 63,153 This approach could enhance our knowledge of the impact of uterine contractions before and during labour, allow the derivation of information on placental capacity and uterine power, and help to provide useful information prior to delivery.
Despite promise, these approaches often require offline processing and significant computational power to produce novel parameter maps presenting quantitative information back to the clinician but have the potential to bring new markers to the clinic.
Computational modelling
While MRI (and other imaging modalities) can assess structural and functional changes between individuals and populations with different pregnancy complications, the multifactorial contributors to fetal health during pregnancy mean that additional insights into the connection between placental structure and function could be invaluable to interpreting imaging going forward.
One strategy to achieve this is via mathematical and computational modelling of placental structure and function. Interpretation of MRI has long been guided by mathematical models, for example, compartmental models of tissue/blood in diffusion weighted imaging. However, personalised modelling approaches are emerging that allow for anatomical data in an individual to be used to predict their placental function. These models could potentially be used to improve analysis of acquired MRI images, and to test hypotheses around what drives function in an individual pregnancy or group of pregnancies, better enabling identification of target “features” in imaging. There are a number of studies that have assessed the morphology of the post-delivery placenta in 3D, with micro-CT imaging emerging as a useful tool to do this. 70,71,154
Whole organ models of the placenta have emerged that aim to describe how blood is distributed throughout the placenta, given the anatomical structure of the blood vessels (size and 3D distribution) that reside within it. 144,154,155 These models treat individual blood vessels as elements that are resistive to flow, and this allows an electric circuit analogy to be applied to predict the distribution of flow and blood pressure within the system. As has recently been demonstrated in rodent models, this analogy can be taken further to predict functional Doppler resulting from the anatomical structure of blood vessels within an organ. 156 This type of modelling provides an opportunity to provide new knowledge on how clinical ultrasound, MRI and functional anatomy relate. Byrne et al 154 demonstrated how variation in anatomy, even at the larger chorionic artery level, leads to significant heterogeneity in blood flow distribution in the feto-placental unit. This heterogeneity was proposed to relate to the capacity of the placenta, i.e. a placenta may function normally, but the heterogeneity in its function may place the placenta at higher risk of a loss of function if part of the placenta experiences a pathological impact. Heterogeneity in structure and function has been observed across MRI studies, particularly in fetal growth restriction, and computational modelling provides an avenue to interpret this heterogeneity in function in terms of risk to fetal blood or oxygen 157–163
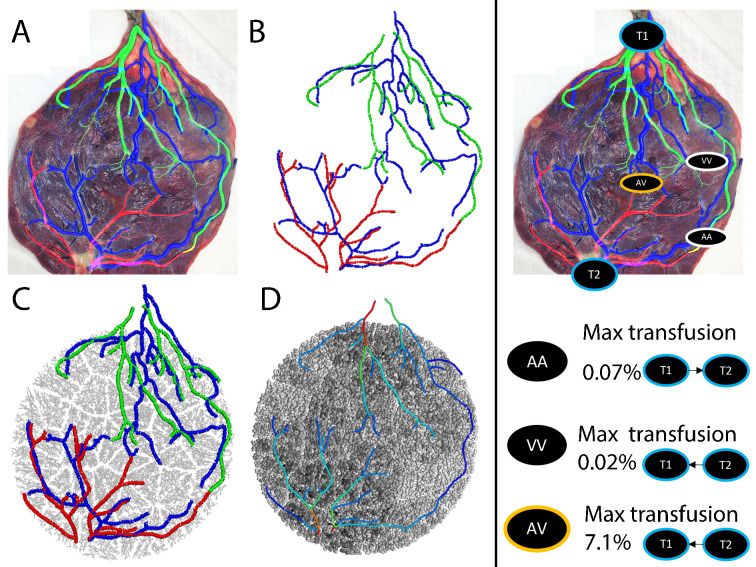
To illustrate how computational modelling could be used to guide surgical planning in TTTS, the model of Byrne et al 69 is applied to assess the chorionic vasculature of a mono-chorionic twin pregnancy in Figure 7. In this case chorionic arteries and veins were derived from photographs of the placenta obtained after delivery, however, these maps could potentially be derived in treatment planning from ultrasound or MRI [. 122,136,137 In this type of model, segmented vascular maps are converted to a graph-like network of nodes and elements, within which blood flow is simulated using Poiseuille’s Law along with conservation of mass at bifurcations. 144 The distribution of blood flow between the two placentas can then be simulated in the absence of any anastomoses, and then in a systematic manner by adding and removing anastomoses to the model. There are three types of anastomoses to consider: true arterio-arterial (AA) anastomoses and veno-venous (VV) anastomoses allow immediate and bi-directional flow and pulse propagation between the circulatory systems of each twin; arterio-venous (AV) anastomoses are implicitly uni-directional, the arterial supply from one twin drains into the venous output of the second, implying that the placental tissue in these instances is shared between twins. 121,164 In this example, the imaged placenta contained arteries and veins from each twin that came in close proximity, but with no anastomosis visible on the choronic surface, hence the AV anastomosis applied was artificial. Model predictions are consistent with the hypotheses that the emergence of TTTS or TAPS is linked to a relative imbalance in the number and size of these anastomoses; net transfusion from AV anastomoses is not compensated by true AA or VV anastomoses 165–167 The strength of the use of computational models in this area is in their ability to assess this net transfusion from anatomical data that may be available in surgical planning on a case-by-case basis.
Figure 7.
A computational model of a twin placenta derived from a map of arteries and veins on the placental chorionic surface. Left panel: (a) Arteries and veins are segmented and (b) their centerlines are converted to a series of connected nodes and elements (in a graph-like structure)[71]. (c) this graph like structure is mapped to a 3D model of the placenta derived from an ellipsoidal fit to the placental boundary[143] (Clark et al. 2015). (d) Blood flow can then be simulated in the major chorionic vessels (colourmap red = 150 ml/min, blue = 1 ml/min) as well as in the gas exchange tissue of the placenta (colourmap black = 0.05 ml/min, white = 0 ml/min). Right panel: Visible arterio-arterial (AA), veno-venous (VV) and arterio-venous (AV) anastomoses can be visualised and be included/removed from the model systematically to assess their individual impact on twin-twin transfusion. In this example, the AA and VV anastomoses have a small contribution, but the AV anastomosis is predicted to transfuse up to 7% of blood flow from twin 2 (T2) to twin 1 (T1). Note that this placenta is from a pregnancy with normal outcome and does not have a clearly identified AV anastomosis, so the addition of this connection is artificial.
Conclusion
This review has presented a summary of current functional imaging techniques for the placenta and some of the computational imaging technology being used to extract individualised placental models. These are likely to play a growing role in imaging developments, although we are still some way from the validation required to bring them to the clinical setting. The potential for improving our understanding of the most interesting of organs and the ability to advance clinical management make this an exciting time for placental research.
Contributor Information
Alys Clark, Email: alys.clark@auckland.ac.nz.
Dimitra Flouri, Email: dimitra.flouri@kcl.ac.uk.
Nada Mufti, Email: n.mufti@ucl.ac.uk.
Joanna James, Email: j.james@auckland.ac.nz.
Eleanor Clements, Email: eleanor.clements@kcl.ac.uk.
Rosalind Aughwane, Email: r.aughwane@ucl.ac.uk.
Michael Aertsen, Email: michael.aertsen@uzleuven.be.
Anna David, Email: a.david@ucl.ac.uk.
Andrew Melbourne, Email: andrew.melbourne@kcl.ac.uk.
REFERENCES
- 1. Long EC. The placenta in lore and legend. Bull Med Libr Assoc 1963; 51: 233–41. [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts RM, Green JA, Schulz LC. The evolution of the placenta. Reproduction 2016; 152: R179-89. doi: 10.1530/REP-16-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wooding P, Burton G. Comparative Placentation. Berlin, Heidelberg: Springer; 2008. doi: 10.1007/978-3-540-78797-6 [DOI] [Google Scholar]
- 4. Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci 2015; 370: 20140066: 20140066. doi: 10.1098/rstb.2014.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol 2018; 218: S745–61: S0002-9378(17)32344-X. doi: 10.1016/j.ajog.2017.11.577 [DOI] [PubMed] [Google Scholar]
- 6. James JL, Chamley LW, Clark AR. Feeding your baby in utero: how the uteroplacental circulation impacts pregnancy. Physiology (Bethesda) 2017; 32: 234–45. doi: 10.1152/physiol.00033.2016 [DOI] [PubMed] [Google Scholar]
- 7. Huppertz B. Traditional and new routes of trophoblast invasion and their implications for pregnancy diseases. Int J Mol Sci 2019; 21(): E289. doi: 10.3390/ijms21010289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allerkamp HH, Clark AR, Lee TC, Morgan TK, Burton GJ, et al. Something old, something new: digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation. Hum Reprod 2021; 36: 571–86. doi: 10.1093/humrep/deaa303 [DOI] [PubMed] [Google Scholar]
- 9. Nardozza LMM, Caetano ACR, Zamarian ACP, Mazzola JB, Silva CP, et al. Fetal growth restriction: current knowledge. Arch Gynecol Obstet 2017; 295: 1061–77. doi: 10.1007/s00404-017-4341-9 [DOI] [PubMed] [Google Scholar]
- 10. Sun C, Groom KM, Oyston C, Chamley LW, Clark AR, et al. The placenta in fetal growth restriction: what is going wrong? Placenta 2020; 96: 10–18. doi: 10.1016/j.placenta.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 11. James JL, Whitley GS, Cartwright JE. Pre-eclampsia: fitting together the placental, immune and cardiovascular pieces. J Pathol 2010; 221: 363–78. doi: 10.1002/path.2719 [DOI] [PubMed] [Google Scholar]
- 12. Kapoor H, Hanaoka M, Dawkins A, Khurana A. Review of MRI imaging for placenta accreta spectrum: pathophysiologic insights, imaging signs, and recent developments. Placenta 2021; 104: 31–39: S0143-4004(20)30443-4. doi: 10.1016/j.placenta.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 13. Society for Maternal-Fetal Medicine, Simpson LL. Twin-twin transfusion syndrome. Am J Obstet Gynecol 2013; 208: 3–18: S0002-9378(12)01980-1. doi: 10.1016/j.ajog.2012.10.880 [DOI] [PubMed] [Google Scholar]
- 14. Barati M, Shahbazian N, Ahmadi L, Masihi S. Diagnostic evaluation of uterine artery doppler sonography for the prediction of adverse pregnancy outcomes. J Res Med Sci 2014; 19: 515–19. [PMC free article] [PubMed] [Google Scholar]
- 15. Yu CKH, Khouri O, Onwudiwe N, Spiliopoulos Y, Nicolaides KH, et al. Prediction of pre-eclampsia by uterine artery doppler imaging: relationship to gestational age at delivery and small-for-gestational age. Ultrasound Obstet Gynecol 2008; 31: 310–13. doi: 10.1002/uog.5252 [DOI] [PubMed] [Google Scholar]
- 16. Llurba E, Carreras E, Gratacós E, Juan M, Astor J, et al. Maternal history and uterine artery doppler in the assessment of risk for development of early- and late-onset preeclampsia and intrauterine growth restriction. Obstet Gynecol Int 2009; 2009: 275613. doi: 10.1155/2009/275613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Llurba E, Carreras E, Gratacós E, Juan M, Astor J, et al. Maternal history and uterine artery doppler in the assessment of risk for development of early- and late-onset preeclampsia and intrauterine growth restriction. Obstet Gynecol Int 2009; 2009: 1–6: 275613. doi: 10.1155/2009/275613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, et al. ISUOG practice guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol 2020; 56: 298–312. doi: 10.1002/uog.22134 [DOI] [PubMed] [Google Scholar]
- 19. Fox H, Sebire NJ. Pathology of the Placenta. 3rd ed. Saunders Elsevier; 2007. [Google Scholar]
- 20. Adamson SL. Arterial pressure, vascular input impedance, and resistance as determinants of pulsatile blood flow in the umbilical artery. Eur J Obstet Gynecol Reprod Biol 1999; 84: 119–25. doi: 10.1016/s0301-2115(98)00320-0 [DOI] [PubMed] [Google Scholar]
- 21. Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol 1989; 161: 1055–60. doi: 10.1016/0002-9378(89)90783-7 [DOI] [PubMed] [Google Scholar]
- 22. Pearce W. Hypoxic regulation of the fetal cerebral circulation. Journal of Applied Physiology 2006; 100: 731–38. doi: 10.1152/japplphysiol.00990.2005 [DOI] [PubMed] [Google Scholar]
- 23. Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol 2005; 25: 32–36. doi: 10.1002/uog.1785 [DOI] [PubMed] [Google Scholar]
- 24. Mathewlynn S, Collins SL. Volume and vascularity: using ultrasound to unlock the secrets of the first trimester placenta. Placenta 2019; 84: 32–36. doi: 10.1016/j.placenta.2019.06.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheung W, Stevenson GN, de Melo Tavares Ferreira AEG, Alphonse J, Welsh AW. Feasibility of image registration and fusion for evaluation of structure and perfusion of the entire second trimester placenta by three-dimensional power doppler ultrasound. Placenta 2020; 94: 13–19. doi: 10.1016/j.placenta.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 26. Noguchi J, Hata K, Tanaka H, Hata T. Placental vascular sonobiopsy using three-dimensional power doppler ultrasound in normal and growth restricted fetuses. Placenta 2009; 30: 391–97. doi: 10.1016/j.placenta.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 27. Collins SL, Stevenson GN, Noble JA, Impey L, Welsh AW. influence of power doppler gain setting on virtual organ computer-aided analysis indices in vivo : can use of the individual sub-noise gain level optimize information? . Ultrasound Obstet Gynecol 2012; 40: 75–80. doi: 10.1002/uog.10122 [DOI] [PubMed] [Google Scholar]
- 28. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 2016; 316: 952. doi: 10.1001/jama.2016.12126 [DOI] [PubMed] [Google Scholar]
- 29. Lum M, Tsiouris AJ. MRI safety considerations during pregnancy. Clinical Imaging 2020; 62: 69–75. doi: 10.1016/j.clinimag.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 30. Alyami W, Kyme A, Bourne R. Histological validation of MRI: A review of challenges in registration of imaging and whole‐mount histopathology. Magnetic Resonance Imaging 2020; 55: 11–22. doi: 10.1002/jmri.27409 [DOI] [PubMed] [Google Scholar]
- 31. Siauve N, Chalouhi GE, Deloison B, Alison M, Clement O, et al. Functional imaging of the human placenta with magnetic resonance. American Journal of Obstetrics and Gynecology 2015; 213: S103–14. doi: 10.1016/j.ajog.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 32. Millischer AE, Deloison B, Silvera S, Ville Y, Boddaert N, et al. Dynamic contrast enhanced MRI of the placenta: A tool for prenatal diagnosis of placenta accreta? Placenta 2017; 53: 40–47. doi: 10.1016/j.placenta.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 33. Jacquier M, Arthuis C, Grévent D, Bussières L, Henry C, et al. Dynamic contrast enhanced magnetic resonance imaging: a review of its application in the assessment of placental function. Placenta 2021; 114: 90–99. doi: 10.1016/j.placenta.2021.08.055 [DOI] [PubMed] [Google Scholar]
- 34. Chalouhi GE, Deloison B, Siauve N, Aimot S, Balvay D, et al. Dynamic contrast-enhanced magnetic resonance imaging: definitive imaging of placental function? Seminars in Fetal and Neonatal Medicine 2011; 16: 22–28. doi: 10.1016/j.siny.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 35. Marcos HB, Semelka RC, Worawattanakul S. Normal placenta: gadolinium-enhanced dynamic MR imaging. Radiology 1997; 205: 493–96. doi: 10.1148/radiology.205.2.9356634 [DOI] [PubMed] [Google Scholar]
- 36. Lo J, Schabel M, Roberts V, Frias A. 106: novel MRI detection of impaired placental blood flow and fetal oxygen reserve in a non-human primate model of gestational protein restriction. American Journal of Obstetrics and Gynecology 2018; 218: S77–78. doi: 10.1016/j.ajog.2017.10.517 [DOI] [Google Scholar]
- 37. Frias AE, Schabel MC, Roberts VHJ, Tudorica A, Grigsby PL, et al. Using dynamic contrast-enhanced MRI to quantitatively characterize maternal vascular organization in the primate placenta. Magn Reson Med 2015; 73: 1570–78. doi: 10.1002/mrm.25264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chalouhi GE, Alison M, Deloison B, Thiam R, Autret G, et al. Fetoplacental oxygenation in an intrauterine growth restriction rat model by using blood oxygen level-dependent MR imaging at 4.7 T. Radiology 2013; 269: 122–29. doi: 10.1148/radiol.13121742 [DOI] [PubMed] [Google Scholar]
- 39. Barbaux S, Erwich JJHM, Favaron PO, Gil S, Gallot D, et al. IFPA meeting 2014 workshop report: animal models to study pregnancy pathologies; new approaches to study human placental exposure to xenobiotics; biomarkers of pregnancy pathologies; placental genetics and epigenetics; the placenta and stillbirth and fetal growth restriction. Placenta 2015; 36 Suppl 1: S5-10: S0143-4004(15)00226-X. doi: 10.1016/j.placenta.2015.01.196 [DOI] [PubMed] [Google Scholar]
- 40. Alison M, Quibel T, Balvay D, Autret G, Bourillon C, et al. Measurement of placental perfusion by dynamic contrast-enhanced MRI at 4.7 T. Invest Radiol 2013; 48: 535–42. doi: 10.1097/RLI.0b013e3182856a25 [DOI] [PubMed] [Google Scholar]
- 41. L.J. Salomon, N. Siauve, F. Taillieu, D. Balvay, C. Vayssettes, G. Frija, Y. Ville, C.A. Cuénod, O . Clément. In Vivo Dynamic MRI Measurement of the Noradrenaline-Induced Reduction in Placental Blood Flow in Mice, Placenta 2006. doi: 10.1016/j.placenta.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 42. Solomon E, Avni R, Hadas R, Raz T, Garbow JR, et al. Major mouse placental compartments revealed by diffusion-weighted MRI, contrast-enhanced MRI, and fluorescence imaging. Proc Natl Acad Sci U S A 2014; 111: 10353–58. doi: 10.1073/pnas.1401695111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo BJ, Yang ZL, Zhang LJ. Gadolinium deposition in brain: current scientific evidence and future perspectives. Front Mol Neurosci 2018; 11: 335. doi: 10.3389/fnmol.2018.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brunelli R, Masselli G, Parasassi T, Spirito MD, Papi M, et al. Intervillous circulation in intra-uterine growth restriction. Correlation to Fetal Well Being, Placenta 2010; 31: 1051–56. doi: 10.1016/j.placenta.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 45. Committee opinion no. 723 summary: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol 2017; 130: 933–34. doi: 10.1097/AOG.0000000000002350 [DOI] [PubMed] [Google Scholar]
- 46. Zun Z, Limperopoulos C. Placental perfusion imaging using velocity-selective arterial spin labeling. Magn Reson Med 2018; 80: 1036–47. doi: 10.1002/mrm.27100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shao X, Liu D, Martin T, Chanlaw T, Devaskar SU, et al. Measuring human placental blood flow with multidelay 3D GRASE pseudocontinuous arterial spin labeling at 3T. J Magn Reson Imaging 2018; 47: 1667–76. doi: 10.1002/jmri.25893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hutter J, Harteveld AA, Jackson LH, Franklin S, Bos C, et al. Perfusion and apparent oxygenation in the human placenta (PERFOX). Magn Reson Med 2020; 83: 549–60. doi: 10.1002/mrm.27950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siauve N, Chalouhi GE, Deloison B, Alison M, Clement O, et al. Functional imaging of the human placenta with magnetic resonance. Am J Obstet Gynecol 2015; 213: S103-14: S0002-9378(15)00658-4. doi: 10.1016/j.ajog.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 50. Woods JG, Chappell MA, Okell TW. A general framework for optimizing arterial spin labeling MRI experiments. Magn Reson Med 2019; 81: 2474–88. doi: 10.1002/mrm.27580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ourselin S, Joskowicz L, Sabuncu MR, Unal G, Wells W. Medical Image Computing and Computer-Assisted Intervention - MICCAI 2016. In: Optimisation of arterial spin labelling using bayesian experimental design. Cham: MICCAI; 2016., pp. 511–18. doi: 10.1007/978-3-319-46726-9 [DOI] [Google Scholar]
- 52. Melbourne A, Aughwane R, Sokolska M, Owen D, Kendall G, et al. Separating fetal and maternal placenta circulations using multiparametric MRI. Magn Reson Med 2019; 81: 350–61. doi: 10.1002/mrm.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168: 497–505. doi: 10.1148/radiology.168.2.3393671 [DOI] [PubMed] [Google Scholar]
- 54. Melbourne A. On the use of multicompartment models of diffusion and relaxation for placental imaging. Placenta 2021; 112: 197–203: S0143-4004(21)00497-5. doi: 10.1016/j.placenta.2021.07.302 [DOI] [PubMed] [Google Scholar]
- 55. Moore RJ, Strachan BK, Tyler DJ, Duncan KR, Baker PN, et al. In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo-planar MRI. Placenta 2000; 21: 726–32. doi: 10.1053/plac.2000.0567 [DOI] [PubMed] [Google Scholar]
- 56. Ong SS, Tyler DJ, Moore RJ, Gowland PA, Baker PN, et al. Functional magnetic resonance imaging (magnetization transfer) and stereological analysis of human placentae in normal pregnancy and in pre-eclampsia and intrauterine growth restriction. Placenta 2004; 25: 408–12. doi: 10.1016/j.placenta.2003.10.011 [DOI] [PubMed] [Google Scholar]
- 57. Shen D, Liu T, Peters TM, Staib LH, Essert C, et al. Medical Image Computing and Computer Assisted Intervention – MICCAI 2019 [In: Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics]. Cham; 2019. doi: 10.1007/978-3-030-32248-9 [DOI] [Google Scholar]
- 58. Couper S, Clark A, Thompson JMD, Flouri D, Aughwane R, et al. The effects of maternal position, in late gestation pregnancy, on placental blood flow and oxygenation: an MRI study. J Physiol 2021; 599: 1901–15. doi: 10.1113/JP280569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Flouri D, Darby JRT, Holman SL, Perumal SR, David AL, et al. Magnetic resonance imaging of placentome development in the pregnant ewe. Placenta 2021; 105: 61–69: S0143-4004(21)00025-4. doi: 10.1016/j.placenta.2021.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hütel M, Antonelli M, Melbourne A, Ourselin S. Hemodynamic matrix factorization for functional magnetic resonance imaging. Neuroimage 2021; 231: : S1053-8119(21)00091-4. doi: 10.1016/j.neuroimage.2021.117814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Uğurbil K, Adriany G, Andersen P, Chen W, Gruetter R, et al. Magnetic resonance studies of brain function and neurochemistry. Annu Rev Biomed Eng 2000; 2: 633–60. doi: 10.1146/annurev.bioeng.2.1.633 [DOI] [PubMed] [Google Scholar]
- 62. Kim SG, Ogawa S. Biophysical and physiological origins of blood oxygenation level-dependent fmri signals. J Cereb Blood Flow Metab 2012; 32: 1188–1206. doi: 10.1038/jcbfm.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sinding M, Peters DA, Frøkjær JB, Christiansen OB, Uldbjerg N, et al. Reduced placental oxygenation during subclinical uterine contractions as assessed by BOLD MRI. Placenta 2016; 39: 16–20: S0143-4004(15)30114-4. doi: 10.1016/j.placenta.2015.12.018 [DOI] [PubMed] [Google Scholar]
- 64. Sinding M, Peters DA, Frøkjær JB, Christiansen OB, Petersen A, et al. Prediction of low birth weight: comparison of placental T2* estimated by MRI and uterine artery pulsatility index. Placenta 2017; 49: 48–54: S0143-4004(16)30639-7. doi: 10.1016/j.placenta.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 65. Sinding M, Peters DA, Poulsen SS, Frøkjær JB, Christiansen OB, et al. Placental baseline conditions modulate the hyperoxic BOLD-MRI response. Placenta 2018; 61: 17–23: S0143-4004(17)31172-4. doi: 10.1016/j.placenta.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 66. Luo J, Abaci Turk E, Bibbo C, Gagoski B, Roberts DJ, et al. In vivo quantification of placental insufficiency by BOLD MRI: A human study. Sci Rep 2017; 7(): 3713. doi: 10.1038/s41598-017-03450-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abaci Turk E, Abulnaga SM, Luo J, Stout JN, Feldman HA, et al. Placental MRI: effect of maternal position and uterine contractions on placental BOLD MRI measurements. Placenta 2020; 95: 69–77. doi: 10.1016/j.placenta.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Plitman Mayo R, Charnock-Jones DS, Burton GJ, Oyen ML. Three-dimensional modeling of human placental terminal villi. Placenta 2016; 43: 54–60. doi: 10.1016/j.placenta.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 69. Byrne M, Aughwane R, James JL, Hutchinson JC, Arthurs OJ, et al. Structure-function relationships in the feto-placental circulation from in silico interpretation of micro-CT vascular structures. J Theor Biol 2021; 517: 110630: S0022-5193(21)00052-7. doi: 10.1016/j.jtbi.2021.110630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Aughwane R, Schaaf C, Hutchinson JC, Virasami A, Zuluaga MA, et al. Micro-CT and histological investigation of the spatial pattern of feto-placental vascular density. Placenta 2019; 88: 36–43: S0143-4004(19)30672-1. doi: 10.1016/j.placenta.2019.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. James JL, Tongpob Y, Srinivasan V, Crew RC, Bappoo N, et al. Three-dimensional visualisation of the feto-placental vasculature in humans and rodents. Placenta 2021; 114: 8–13: S0143-4004(21)00547-6. doi: 10.1016/j.placenta.2021.08.049 [DOI] [PubMed] [Google Scholar]
- 72. Jirkovská M, Kubínová L, Krekule I, Hach P. Spatial arrangement of fetal placental capillaries in terminal villi: a study using confocal microscopy. Anat Embryol (Berl) 1998; 197: 263–72. doi: 10.1007/s004290050136 [DOI] [PubMed] [Google Scholar]
- 73. Resta L, Capobianco C, Marzullo A, Piscitelli D, Sanguedolce F, et al. Confocal laser scanning microscope study of terminal villi vessels in normal term and pre-eclamptic placentas. Placenta 2006; 27: 735–39. doi: 10.1016/j.placenta.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 74. Klimowicz E, Shah R, Girardi T, Lederman S, Dygulska B, et al. Placental chorionic vascular angiogenesis is altered in pre-eclampsia and fetal growth restriction independent of gestational age. Placenta 2017; 57: 270. doi: 10.1016/j.placenta.2017.07.155 [DOI] [Google Scholar]
- 75. Aughwane R, Ingram E, Johnstone ED, Salomon LJ, David AL, et al. Placental MRI and its application to fetal intervention. Prenatal Diagnosis 2019; 40: 38–48. doi: 10.1002/pd.5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Klimowicz E, Shah R, Girardi T, Lederman S, Dygulska B, et al. Placental chorionic vascular angiogenesis is altered in pre-eclampsia and fetal growth restriction independent of gestational age. Placenta 2017; 57: 270. doi: 10.1016/j.placenta.2017.07.155 [DOI] [Google Scholar]
- 77. Lu T, Pu H, Cui W, Mei J, Huang M, et al. Use of intravoxel incoherent motion MR imaging to assess placental perfusion in patients with placental adhesion disorder on their third trimester. Clinical Imaging 2019; 56: 135–39. doi: 10.1016/j.clinimag.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 78. Kapoor H, Hanaoka M, Dawkins A, Khurana A. Review of MRI imaging for placenta accreta spectrum: pathophysiologic insights, imaging signs, and recent developments. Placenta 2021; 104: 31–39. doi: 10.1016/j.placenta.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 79. Hutter J, Jackson L, Ho A, Avena Zampieri C, Hajnal JV, et al. The use of functional placental magnetic resonance imaging for assessment of the placenta after prolonged preterm rupture of the membranes in vivo: A pilot study. Acta Obstet Gynecol Scand 2021; 100: 2244–52. doi: 10.1111/aogs.14267 [DOI] [PubMed] [Google Scholar]
- 80. De Paepe ME, Shapiro S, Young L, Luks FI. Placental characteristics of selective birth weight discordance in diamniotic-monochorionic twin gestations. Placenta 2010; 31: 380–86. doi: 10.1016/j.placenta.2010.02.018 [DOI] [PubMed] [Google Scholar]
- 81. Wee LY, Sebire NJ, Bhundia J, Sullivan M, Fisk NM. Histomorphometric characterisation of shared and non-shared cotyledonary villus territories of monochorionic placentae in relation to pregnancy complications. Placenta 2006; 27: 475–82. doi: 10.1016/j.placenta.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 82. Fisk NM, Duncombe GJ, Sullivan MHF. The basic and clinical science of twin–twin transfusion syndrome. Placenta 2009; 30: 379–90. doi: 10.1016/j.placenta.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 83. Crispi F, Miranda J, Gratacós E. Long-term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. American Journal of Obstetrics and Gynecology 2018; 218: S869–79. doi: 10.1016/j.ajog.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 84. Murray E, Fernandes M, Fazel M, Kennedy S, Villar J, et al. Differential effect of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG: Int J Obstet Gy 2015; 122: 1062–72. doi: 10.1111/1471-0528.13435 [DOI] [PubMed] [Google Scholar]
- 85. Reinebrant HE, Leisher SH, Coory M, Henry S, Wojcieszek AM, et al. Making stillbirths visible: a systematic review of globally reported causes of stillbirth. BJOG 2018; 125: 212–24. doi: 10.1111/1471-0528.14971 [DOI] [PubMed] [Google Scholar]
- 86. Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, et al. Stillbirths: where? when? why? how to make the data count? Lancet 2011; 377: 1448–63. doi: 10.1016/S0140-6736(10)62187-3 [DOI] [PubMed] [Google Scholar]
- 87. Benton SJ, McCowan LM, Heazell AEP, Grynspan D, Hutcheon JA, et al. Placental growth factor as a marker of fetal growth restriction caused by placental dysfunction. Placenta 2016; 42: 1–8: S0143-4004(16)30046-7. doi: 10.1016/j.placenta.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 88. Kingdom JC, Audette MC, Hobson SR, Windrim RC, Morgen E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obstet Gynecol 2018; 218: S803–17: S0002-9378(17)32340-2. doi: 10.1016/j.ajog.2017.11.575 [DOI] [PubMed] [Google Scholar]
- 89. Ingram E, Naish J, Morris DM, Myers J, Johnstone ED. 53: MRI measurements of abnormal placental oxygenation in pregnancies complicated by FGR. American Journal of Obstetrics and Gynecology 2018; 218: S40–41. doi: 10.1016/j.ajog.2017.10.464 [DOI] [Google Scholar]
- 90. Aughwane R, Mufti N, Flouri D, Maksym K, Spencer R, et al. Magnetic resonance imaging measurement of placental perfusion and oxygen saturation in early‐onset fetal growth restriction. BJOG: Int J Obstet Gy 2020; 128: 337–45. doi: 10.1111/1471-0528.16387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hutter J, Slator PJ, Jackson L, Gomes ADS, Ho A, et al. Multi-modal functional mri to explore placental function over gestation. Magn Reson Med 2019; 81: 1191–1204. doi: 10.1002/mrm.27447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aughwane R, Mufti N, Flouri D, Maksym K, Spencer R, et al. Magnetic resonance imaging measurement of placental perfusion and oxygen saturation in early‐onset fetal growth restriction. BJOG: Int J Obstet Gy 2020; 128: 337–45. doi: 10.1111/1471-0528.16387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Derwig I, Barker GJ, Poon L, Zelaya F, Gowland P, et al. Association of placental t2 relaxation times and uterine artery doppler ultrasound measures of placental blood flow. Placenta 2013; 34: 474–79. doi: 10.1016/j.placenta.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 94. Bonel HM, Stolz B, Diedrichsen L, Frei K, Saar B, et al. Diffusion-weighted MR imaging of the placenta in fetuses with placental insufficiency. Radiology 2010; 257: 810–19. doi: 10.1148/radiol.10092283 [DOI] [PubMed] [Google Scholar]
- 95. Song F, Wu W, Qian Z, Zhang G, Cheng Y. Assessment of the placenta in intrauterine growth restriction by diffusion-weighted imaging and proton magnetic resonance spectroscopy. Reprod Sci 2016; 24: 575–81. doi: 10.1177/1933719116667219 [DOI] [PubMed] [Google Scholar]
- 96. Derwig I, Lythgoe DJ, Barker GJ, Poon L, Gowland P, et al. Association of placental perfusion, as assessed by magnetic resonance imaging and uterine artery doppler ultrasound, and its relationship to pregnancy outcome. Placenta 2013; 34: 885–91: S0143-4004(13)00593-6. doi: 10.1016/j.placenta.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 97. Siauve N, Hayot PH, Deloison B, Chalouhi GE, Alison M, et al. Assessment of human placental perfusion by intravoxel incoherent motion MR imaging. J Matern Fetal Neonatal Med 2019; 32: 293–300. doi: 10.1080/14767058.2017.1378334 [DOI] [PubMed] [Google Scholar]
- 98. Aimot-Macron S, Salomon LJ, Deloison B, Thiam R, Cuenod CA, et al. In vivo MRI assessment of placental and foetal oxygenation changes in a rat model of growth restriction using blood oxygen level-dependent (BOLD) magnetic resonance imaging. Eur Radiol 2013; 23: 1335–42. doi: 10.1007/s00330-012-2712-y [DOI] [PubMed] [Google Scholar]
- 99. Cronin RS, Li M, Thompson JMD, Gordon A, Raynes-Greenow CH, et al. An individual participant data meta-analysis of maternal going-to-sleep position, interactions with fetal vulnerability, and the risk of late stillbirth. [Interactions with Fetal Vulnerability, and the Risk of Late Stillbirth]. EClinicalMedicine 2019; 10: 49–57. doi: 10.1016/j.eclinm.2019.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Humphries A, Mirjalili SA, Tarr GP, Thompson JMD, Stone P. The effect of supine positioning on maternal hemodynamics during late pregnancy. J Matern Fetal Neonatal Med 2019; 32: 3923–30. doi: 10.1080/14767058.2018.1478958 [DOI] [PubMed] [Google Scholar]
- 101. Jauniaux E, Ayres‐de‐Campos D, Langhoff‐Roos J, Fox KA, Collins S, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders, . Int J Gynecol Obstet 2019; 146: 20–24. doi: 10.1002/ijgo.12761 [DOI] [PubMed] [Google Scholar]
- 102. Jauniaux E, Bhide A, Kennedy A, Woodward P, Hubinont C, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: prenatal diagnosis and screening. Int J Gynaecol Obstet 2018; 140: 274–80. doi: 10.1002/ijgo.12408 [DOI] [PubMed] [Google Scholar]
- 103. Jauniaux E, Grønbeck L, Bunce C, Langhoff-Roos J, Collins SL. Epidemiology of placenta previa accreta: A systematic review and meta-analysis. BMJ Open 2019; 9: 1–9: e031193. doi: 10.1136/bmjopen-2019-031193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jauniaux E, Bhide A, Kennedy A, Woodward P, Hubinont C, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: prenatal diagnosis and screening. Int J Gynaecol Obstet 2018; 140: 274–80. doi: 10.1002/ijgo.12408 [DOI] [PubMed] [Google Scholar]
- 105. Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta 2012; 33: 244–51. doi: 10.1016/j.placenta.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 106. Cuthbert F, Teixidor Vinas M, Whitby E. The MRI features of placental adhesion disorder-a pictorial review. Br J Radiol 2016; 89(): 20160284. doi: 10.1259/bjr.20160284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta 2008; 29: 639–45. doi: 10.1016/j.placenta.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 108. Shetty MK, Dryden DK. Morbidly adherent placenta: ultrasound assessment and supplemental role of magnetic resonance imaging. Semin Ultrasound CT MR 2015; 36: 324–31: S0887-2171(15)00042-6. doi: 10.1053/j.sult.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 109. Goergen SK, Posma E, Wrede D, Collett J, Pyman J, et al. Interobserver agreement and diagnostic performance of individual MRI criteria for diagnosis of placental adhesion disorders. Clin Radiol 2018; 73: 908. S0009-9260(18)30214-9. doi: 10.1016/j.crad.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 110. D’Antonio F, Iacovella C, Palacios-Jaraquemada J, Bruno CH, Manzoli L, et al. Prenatal identification of invasive placentation using magnetic resonance imaging: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2014; 44: 8–16. doi: 10.1002/uog.13327 [DOI] [PubMed] [Google Scholar]
- 111. Collins SL, Alemdar B, van Beekhuizen HJ, Bertholdt C, Braun T, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the international society for abnormally invasive placenta. American Journal of Obstetrics and Gynecology 2019; 220: 511–26. doi: 10.1016/j.ajog.2019.02.054 [DOI] [PubMed] [Google Scholar]
- 112. Collins SL, Ashcroft A, Braun T, Calda P, Langhoff-Roos J, et al. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol 2016; 47: 271–75. doi: 10.1002/uog.14952 [DOI] [PubMed] [Google Scholar]
- 113. Morel O, Collins SL, Uzan-Augui J, Masselli G, Duan J, et al. A proposal for standardized magnetic resonance imaging (MRI) descriptors of abnormally invasive placenta (AIP) – from the international society for AIP. Diagnostic and Interventional Imaging 2019; 100: 319–25. doi: 10.1016/j.diii.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 114. Jha P, Pōder L, Bourgioti C, Bharwani N, Lewis S, et al. Society of abdominal radiology (SAR) and european society of urogenital radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders. Eur Radiol 2020; 30: 2604–15. doi: 10.1007/s00330-019-06617-7 [DOI] [PubMed] [Google Scholar]
- 115. Agostini TCF, Figueiredo R, Warmbrand G, Torres US, Pria HRFD, et al. Placental adhesion disorder: magnetic resonance imaging features and a proposal for a structured report. Radiol Bras 2020; 53: 329–36. doi: 10.1590/0100-3984.2019.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kilcoyne A, Shenoy-Bhangle AS, Roberts DJ, Sisodia RC, Gervais DA, et al. MRI of placenta accreta, placenta increta, and placenta percreta: pearls and pitfalls. AJR Am J Roentgenol 2017; 208: 214–21. doi: 10.2214/AJR.16.16281 [DOI] [PubMed] [Google Scholar]
- 117. Morita S, Ueno E, Fujimura M, Muraoka M, Takagi K, et al. Feasibility of diffusion-weighted MRI for defining placental invasion. J Magn Reson Imaging 2009; 30: 666–71. doi: 10.1002/jmri.21875 [DOI] [PubMed] [Google Scholar]
- 118. Jerome NP, d’Arcy JA, Feiweier T, Koh D-M, Leach MO, et al. Extended T2-IVIM model for correction of TE dependence of pseudo-diffusion volume fraction in clinical diffusion-weighted magnetic resonance imaging. Phys Med Biol 2016; 61: 667–80. doi: 10.1088/1361-6560/61/24/N667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ebner M, Wang G, Li W, Aertsen M, Patel PA, et al. An automated framework for localization, segmentation and super-resolution reconstruction of fetal brain MRI. Neuroimage 2020; 206: : S1053-8119(19)30915-2. doi: 10.1016/j.neuroimage.2019.116324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Uus A, Zhang T, Jackson LH, Roberts TA, Rutherford MA, et al. Deformable slice-to-volume registration for motion correction of fetal body and placenta MRI. IEEE Trans Med Imaging 2020; 39: 2750–59. doi: 10.1109/TMI.2020.2974844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Denbow ML, Cox P, Taylor M, Hammal DM, Fisk NM. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol 2000; 182: 417–26. doi: 10.1016/s0002-9378(00)70233-x [DOI] [PubMed] [Google Scholar]
- 122. Hack KEA, Nikkels PGJ, Koopman-Esseboom C, Derks JB, Elias SG, et al. Placental characteristics of monochorionic diamniotic twin pregnancies in relation to perinatal outcome. Placenta 2008; 29: 976–81. doi: 10.1016/j.placenta.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 123. Roberts D, Neilson JP, Kilby MD, Gates S. Interventions for the treatment of twin-twin transfusion syndrome. Cochrane Database Syst Rev 2014; : CD002073. doi: 10.1002/14651858.CD002073.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lopriore E, Ortibus E, Acosta-Rojas R, Le Cessie S, Middeldorp JM, et al. Risk factors for neurodevelopment impairment in twin-twin transfusion syndrome treated with fetoscopic laser surgery. Obstet Gynecol 2009; 113: 361–66. doi: 10.1097/AOG.0b013e318195873e [DOI] [PubMed] [Google Scholar]
- 125. Spruijt MS, Lopriore E, Tan RNGB, Slaghekke F, Klumper FJCM, et al. Long-term neurodevelopmental outcome in twin-to-twin transfusion syndrome: is there still room for improvement? J Clin Med 2019; 8: 1226: E1226. doi: 10.3390/jcm8081226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Roberts D, Gates S, Kilby M, Neilson JP. Interventions for twin-twin transfusion syndrome: A Cochrane review, Ultrasound in Obstetrics and Gynecology. 2008. doi: 10.1002/uog.5328 [DOI] [PubMed]
- 127. Ruano R, Rodo C, Peiro JL, Shamshirsaz AA, Haeri S, et al. Fetoscopic laser ablation of placental anastomoses in twin-twin transfusion syndrome using ‘solomon technique.’ Ultrasound Obstet Gynecol 2013; n. doi: 10.1002/uog.12492 [DOI] [PubMed] [Google Scholar]
- 128. Baschat A, Oepkes D. Twin anemia-polycythemia sequence in monochorionic twins: implications for diagnosis and treatment. Amer J Perinatol 2014; 31: S25–30. doi: 10.1055/s-0034-1376391 [DOI] [PubMed] [Google Scholar]
- 129. Sepulveda W, Sebire NJ, Hughes K, Odibo A, Nicolaides KH. The lambda sign at 10-14 weeks of gestation as a predictor of chorionicity in twin pregnancies. Ultrasound Obstet Gynecol 1996; 7: 421–23. doi: 10.1046/j.1469-0705.1996.07060421.x [DOI] [PubMed] [Google Scholar]
- 130. Senat M-V, Deprest J, Boulvain M, Paupe A, Winer N, et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med 2004; 351: 136–44. doi: 10.1056/NEJMoa032597 [DOI] [PubMed] [Google Scholar]
- 131. Lewi L, Jani J, Blickstein I, Huber A, Gucciardo L, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. American Journal of Obstetrics and Gynecology 2008; 199: 514. doi: 10.1016/j.ajog.2008.03.050 [DOI] [PubMed] [Google Scholar]
- 132. Yankeelov TE, Luci JJ, Lepage M, Li R, Debusk L, et al. Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: A reference region model. Magn Reson Imaging 2005; 23: 519–29. doi: 10.1016/j.mri.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 133. Couck I, Lewi L. The placenta in twin-to-twin transfusion syndrome and twin anemia polycythemia sequence. Twin Res Hum Genet 2016; 19: 184–90. doi: 10.1017/thg.2016.29 [DOI] [PubMed] [Google Scholar]
- 134. Bajoria R, Wee LY, Anwar S, Ward S. Outcome of twin pregnancies complicated by single intrauterine death in relation to vascular anatomy of the monochorionic placenta. Hum Reprod 1999; 14: 2124–30. doi: 10.1093/humrep/14.8.2124 [DOI] [PubMed] [Google Scholar]
- 135. Lewi L, Jani J, Blickstein I, Huber A, Gucciardo L, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol 2008; 199: 514. doi: 10.1016/j.ajog.2008.03.050 [DOI] [PubMed] [Google Scholar]
- 136. Dellschaft NS, Hutchinson G, Shah S, Jones NW, Bradley C, et al. The haemodynamics of the human placenta in utero. PLoS Biol 2020; 18(): e3000676. doi: 10.1371/journal.pbio.3000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Frangi AF, Schnabel JA, Davatzikos C, Alberola-López C, Fichtinger G. Medical Image Computing and Computer Assisted Intervention – MICCAI 2018 [In: Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics]. Cham; 2018. doi: 10.1007/978-3-030-00928-1 [DOI] [Google Scholar]
- 138. Mufti N, Aertsen M, Ebner M, Fidon L, Patel P, et al. Cortical spectral matching and shape and volume analysis of the fetal brain pre- and post-fetal surgery for spina bifida: a retrospective study. Neuroradiology 2021; 63: 1721–34. doi: 10.1007/s00234-021-02725-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wang G, Zuluaga MA, Pratt R, Aertsen M, Doel T, et al. Slic-seg: A minimally interactive segmentation of the placenta from sparse and motion-corrupted fetal MRI in multiple views. Medical Image Analysis 2016; 34: 137–47. doi: 10.1016/j.media.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pratt R, Hutchinson JC, Melbourne A, Zuluaga MA, Virasami A, et al. Imaging the human placental microcirculation with micro-focus computed tomography: optimisation of tissue preparation and image acquisition. Placenta 2017; 60: 36–39. doi: 10.1016/j.placenta.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Byrne M, Aughwane R, James J, Hutchinson C, Arthurs O, et al. Structure-function relationships in the feto-placental circulation from in silico interpretation of micro-CT vascular structures. Placenta 2019; 83: e84. doi: 10.1016/j.placenta.2019.06.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Flouri D, Owen D, Aughwane R, Mufti N, Maksym K, et al. Improved fetal blood oxygenation and placental estimated measurements of diffusion‐weighted MRI using data‐driven bayesian modeling. Magn Reson Med 2019; 83: 2160–72. doi: 10.1002/mrm.28075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Torrents-Barrena J, López-Velazco R, Piella G, Masoller N, Valenzuela-Alcaraz B, et al. TTTS-GPS: patient-specific preoperative planning and simulation platform for twin-to-twin transfusion syndrome fetal surgery. Computer Methods and Programs in Biomedicine 2019; 179: 104993. doi: 10.1016/j.cmpb.2019.104993 [DOI] [PubMed] [Google Scholar]
- 144. Clark AR, Lin M, Tawhai M, Saghian R, James JL. Multiscale modelling of the feto–placental vasculature. Interface Focus 2015; 5: 20140078. doi: 10.1098/rsfs.2014.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, et al. Sampling and definitions of placental lesions: amsterdam placental workshop group consensus statement. Archives of Pathology & Laboratory Medicine 2016; 140: 698–713. doi: 10.5858/arpa.2015-0225-CC [DOI] [PubMed] [Google Scholar]
- 146. Slator PJ, Hutter J, McCabe L, Gomes ADS, Price AN, et al. Placenta microstructure and microcirculation imaging with diffusion MRI. Magn Reson Med 2018; 80: 756–66. doi: 10.1002/mrm.27036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Melbourne A. On the use of multicompartment models of diffusion and relaxation for placental imaging. Placenta 2021; 112: 197–203: S0143-4004(21)00497-5. doi: 10.1016/j.placenta.2021.07.302 [DOI] [PubMed] [Google Scholar]
- 148. Melbourne A, Aughwane R, Sokolska M, Owen D, Kendall G, et al. Separating fetal and maternal placenta circulations using multiparametric MRI. Magn Reson Med 2019; 81: 350–61. doi: 10.1002/mrm.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bonel HM, Stolz B, Diedrichsen L, Frei K, Saar B, et al. Diffusion-weighted MR imaging of the placenta in fetuses with placental insufficiency. Radiology 2010; 257: 810–19. doi: 10.1148/radiol.10092283 [DOI] [PubMed] [Google Scholar]
- 150. Slator PJ, Hutter J, McCabe L, Gomes ADS, Price AN, et al. Placenta microstructure and microcirculation imaging with diffusion MRI. Magn Reson Med 2018; 80: 756–66. doi: 10.1002/mrm.27036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Couper S, Clark A, Mirjalili A, Flouri D, Aughwane R, et al. Magnetic resonance assessment of the effect of maternal position on fetoplacental blood flow and oxygenation. Placenta 2019; 83: e84. doi: 10.1016/j.placenta.2019.06.268 [DOI] [Google Scholar]
- 152. Osmanski B-F, Lecarpentier E, Montaldo G, Tsatsaris V, Chavatte-Palmer P, et al. Discriminative imaging of maternal and fetal blood flow within the placenta using ultrafast ultrasound. Sci Rep 2015; 5: 1–10: 13394. doi: 10.1038/srep13394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bakker PCAM, Van Rijswijk S, Van Rijsiwijk S, van Geijn HP. Uterine activity monitoring during labor. J Perinat Med 2007; 35: 468–77. doi: 10.1515/JPM.2007.116 [DOI] [PubMed] [Google Scholar]
- 154. Byrne M, Aughwane R, James JL, Hutchinson JC, Arthurs OJ, et al. Structure-function relationships in the feto-placental circulation from in silico interpretation of micro-CT vascular structures. J Theor Biol 2021; 517: 110630: S0022-5193(21)00052-7. doi: 10.1016/j.jtbi.2021.110630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tun WM, Yap CH, Saw SN, James JL, Clark AR. Differences in placental capillary shear stress in fetal growth restriction may affect endothelial cell function and vascular network formation. Sci Rep 2019; 9: 1–10: 9876. doi: 10.1038/s41598-019-46151-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Saghian R, Cahill L, Rahman A, Steinman J, Stortz G, et al. Interpretation of wave reflections in the umbilical arterial segment of the feto-placental circulation: computational modeling of the feto-placental arterial tree. IEEE Trans Biomed Eng 2021; 68: 3647–58. doi: 10.1109/TBME.2021.3082064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chernyavsky IL, Jensen OE, Leach L. A mathematical model of intervillous blood flow in the human placentone. Placenta 2010; 31: 44–52. doi: 10.1016/j.placenta.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 158. Nye GA, Ingram E, Johnstone ED, Jensen OE, Schneider H, et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J Physiol 2018; 596: 5523–34. doi: 10.1113/JP275633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lin M, Mauroy B, James JL, Tawhai MH, Clark AR. A multiscale model of placental oxygen exchange: the effect of villous tree structure on exchange efficiency. J Theor Biol 2016; 408: 1–12: S0022-5193(16)30171-0. doi: 10.1016/j.jtbi.2016.06.037 [DOI] [PubMed] [Google Scholar]
- 160. Pearce P, Brownbill P, Janáček J, Jirkovská M, Kubínová L, et al. Image-based modeling of blood flow and oxygen transfer in feto-placental capillaries. PLoS One 2016; 11(): e0165369. doi: 10.1371/journal.pone.0165369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Plitman Mayo R, Olsthoorn J, Charnock-Jones DS, Burton GJ, Oyen ML. Computational modeling of the structure-function relationship in human placental terminal villi. J Biomech 2016; 49: 3780–87: S0021-9290(16)31061-2. doi: 10.1016/j.jbiomech.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 162. Couck I, Ponnet S, Thewissen L, Russo F, Deprest J, et al. The detection, outcome, and presentation of twin-twin transfusion syndrome in monochorionic diamniotic twin pregnancies followed with a protocol of fortnightly ultrasound examination. Fetal Diagn Ther 2021; 48: 353–60. doi: 10.1159/000514575 [DOI] [PubMed] [Google Scholar]
- 163. Torrents-Barrena J, Piella G, Masoller N, Gratacós E, Eixarch E, et al. Fully automatic 3D reconstruction of the placenta and its peripheral vasculature in intrauterine fetal MRI. Medical Image Analysis 2019; 54: 263–79. doi: 10.1016/j.media.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 164. Lewi L, Schoubroeck DV, Gratacós E, Witters I, Timmerman D, et al. Monochorionic diamniotic twins: complications and management options. Current Opinion in Obstetrics and Gynecology 2003; 15: 177–94. doi: 10.1097/00001703-200304000-00013 [DOI] [PubMed] [Google Scholar]
- 165. Lewi L, Cannie M, Blickstein I, Jani J, Huber A, et al. Placental sharing, birthweight discordance, and vascular anastomoses in monochorionic diamniotic twin placentas. American Journal of Obstetrics and Gynecology 2007; 197: 587. doi: 10.1016/j.ajog.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 166. Lewi L, Gucciardo L, Van Mieghem T, de Koninck P, Beck V, et al. Monochorionic diamniotic twin pregnancies: natural history and risk stratification. Fetal Diagn Ther 2010; 27: 121–33. doi: 10.1159/000313300 [DOI] [PubMed] [Google Scholar]
- 167. Faye-Petersen OM, Crombleholme TM. Twin-to-twin transfusion syndrome. NeoReviews 2008; 9: e370-79. doi: 10.1542/neo.9-9-e370 [DOI] [Google Scholar]