Summary statement:
Gram-negative bacteria are responsible for an increasing number of deaths from antibiotic resistant infections.1,2 The bacterial natural product, colistin, is considered the last line of defense against a number of Gram-negative pathogens. The recent global spread of the plasmid-borne mobilized colistin resistance gene mcr-1 (phosphoethanolamine (PEtN) transferase) threatens colistin’s utility.3 Bacterial-derived antibiotics often appear in nature as collections of similar structures that are encoded by evolutionarily related biosynthetic gene clusters (BGCs). This structural diversity is, at least in part, likely a response to the development of natural resistance, which often mechanistically mimics clinical resistance. Here, we proposed that a solution to mcr-1 mediated resistance might have evolved among naturally occurring colistin congeners. Our search of sequenced bacterial genomes identified a BGC that was predicted to encode the most structurally divergent colistin congener described to-date. Chemical synthesis of this structure produced macolacin, which is active against Gram-negative pathogens containing mcr-1 as well as intrinsically resistant pathogens with chromosomally encoded PEtN transferase genes. These include extremely drug resistant (XDR) Acinetobacter baumannii and intrinsically colistin resistant Neisseria gonorrhoeae which, due to a lack of effective treatment options, are considered among the highest level Gram-negative threats.4 In a mouse neutropenic infection model, a biphenyl analog of macolacin proved to be effective against colistin resistant XDR A. baumannii, thus providing a new naturally inspired and easily produced therapeutic lead for combating colistin resistant pathogens.
Keywords: Antibiotic resistance, mcr-1, synthetic bioinformatic natural product (syn-BNP), Gram-negative bacteria, colistin, Acinetobacter baumannii, Neisseria gonorrhoeae, XDR Gram-negative bacteria
Introduction:
Multidrug-resistant (MDR) Gram-negative bacteria represent a serious and growing risk to public health.1,5 Many critical Gram-negative active antibiotics in use today are either bacterial metabolites or inspired by bacterial metabolites.2,6 In fact, the bacterial natural product colistin is used as the last line of defense against serious infections caused by a number of MDR Gram-negative pathogens, especially those with carbapenem resistance.7,8 Colistin binds the lipid A moiety of lipopolysaccharides (LPS), disrupting membrane integrity, and ultimately causing cell death. Unfortunately, the extensive use of colistin in animal production, and its increasing use in human pharmacotherapy, has led to a troubling rise in resistant clinical isolates.9 Of particular concern is the recent appearance and rapid global spread of the plasmid-borne mobilized colistin resistance (mcr-1) gene and its relatives. Mcr-1 encodes a PEtN transferase that appends PEtN to a phosphate on lipid A thereby reducing the electrostatic interaction between colistin and LPS and rendering bacteria resistant to colistin. Since first being observed in 2015, mcr-1 has been detected around the world in clinical isolates of numerous Gram-negative pathogens.10–13
As is seen for most natural product antibiotics, colistin is part of a collection of structurally related metabolites that are encoded by evolutionarily related but distinct BGCs. Colistin belongs to the polymyxin family of antibiotics, which are cationic cyclic lipo-decapeptides that arise from nonribosomal peptide synthetase (NRPS) BGCs found in the genomes of Paenibacillus spp. Across this family of antibiotics, structures differ slightly by both peptide sequence and the specific lipid that is attached to the N-terminus of the decapeptide. The ecological significance of the evolution of collections of structural analogs in place of a single winning natural antibiotic structure may differ from one family to the next; however, the evolution of antibiotic resistance is undoubtedly one of the key drivers of this structural diversification. While occurring on dramatically different time scales, clinical and environmental associated antibiotic resistance arise from the same pool of potential resistance genes. As a result, naturally occurring congeners, which have evolved to circumvent environmental resistance mechanisms, could prove useful for addressing antibiotic resistance that has evolved in the health care setting (Fig. 1a). In the case of mcr-1 mediated colistin resistance, this possibility was especially intriguing to us as bacteria that are intrinsically resistant to colistin often contain chromosomally encoded PEtN transferases that could, long before the recent global mobilization of mcr-1, have driven the natural selection for colistin like antibiotics that circumvent this lipid A modification. Such an antibiotic would be particularly appealing as it would not only be useful for addressing mcr-1 encoded resistance, but also potentially useful against a number of difficult to treat pathogens that are intrinsically resistant to colistin due to chromosomally encoded PEtN transferases (e.g., N. gonorrohoeae).
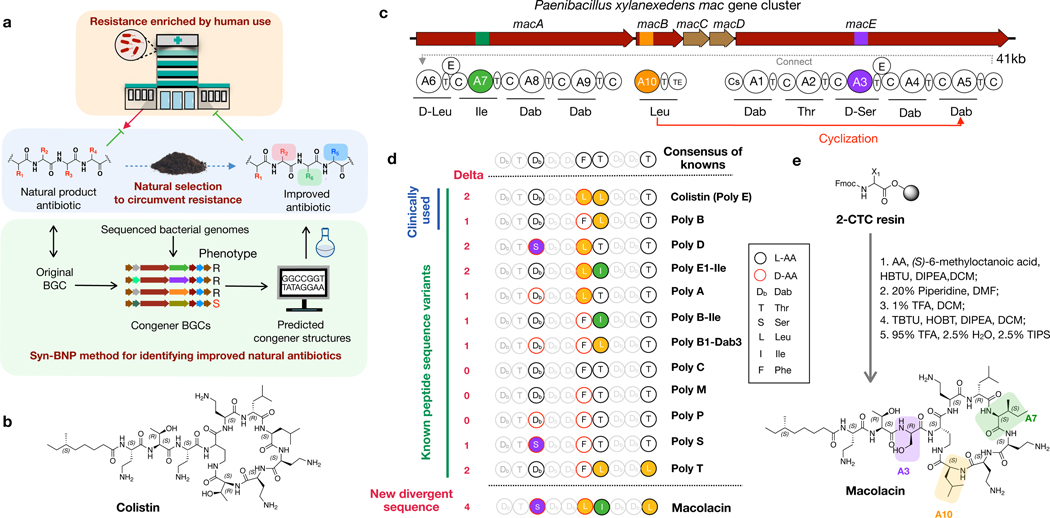
Fig. 1 |. Discovery of macolacin.
a) Extensive use of antibiotics has resulted in the increased appearance of antibiotic resistant pathogens in the clinical setting. In nature, a similar phenomenon is likely occurring in response to the natural production of antibiotics by bacteria. Nature however is not static, and the response to the development of resistance by some bacteria will be the selection of BGCs that encode variants of antibiotics that are capable of circumventing common resistance mechanisms. Here we use BGC guided chemical synthesis to identify a naturally occurring analog of colistin that is active against resistance encoded by the recently identified and now globally distributed mcr-1 gene. b) Structure of colistin. c) The mac gene cluster. The domain structure encoded by NRPS genes macA-macE. NRP synthesis is initiated from the Cs (condensation starter) domain. C (condensation), A (adenylation), and T (thiolation) domains make a minimal NPRS module that extends the growing NRP by one amino acid. Inclusion of an epimerization (E) domain in the module alters the stereochemistry of the T domain bound amino acid. The TE (thioesterase) domain releases the mature NRP from the final T domain. d) Comparison of the predicted macolacin decapeptide to decapeptides found in characterized polymyxin (poly) structures. The number of amino acids that each peptide differs from the consensus peptide derived from all known polymyxin structure is shown (Delta). e) Chemical synthesis of macolacin.
Discovery of macolacin:
Because of the historical difficulties with culturing bacteria as well as difficulties with activating BGCs in laboratory fermentation studies, only a subset of the naturally occurring congeners within a family of antibiotics is likely represented among characterized natural products.14 The recent exponential growth in genomic and metagenomic sequence data provides a window into bacterial BGCs that have until now remained functionally inaccessible in the search for new antibiotics. In an effort to systematically identify naturally occurring polymyxin family members, we searched 10,858 bacterial genomes for polymyxin/colistin-like BGCs (Fig. 1b, c). This led to our identification of 35 BGCs that we predicted would encode polymyxin family antibiotics (Table. S2). Each BGC contains the same gene content and gene organization as is seen in previously characterized polymyxin-family BGCs and each is predicted to encode an N-acylated decapeptide. Nonribosomal peptides (NRPs) are produced by sets of multi domain modules that extend the growing peptide one amino acid per module. A typical minimal NRPS module contains an adenylation (A), a condensation (C) and a thiolation (T) domain, which activate an amino acid substrate, catalyze peptide bond formation and carry the growing peptide, respectively (Fig. 1c).15 The specific amino acid incorporated into the growing NRP by a module can be empirically determined based on the 10 amino acids that line the A-domain substrate binding pocket.16 To determine the linear decapeptide that is produced by each predicted polymyxin family BGC, each A-domain substrate binding pocket was compared to the 10 amino acid signatures seen in a collection of characterized A-domains (Extended Table 1).
Known polymyxin congeners do not differ significantly from the consensus peptide that arises from comparing all characterized antibiotics in the family.17,18 Based on our A-domain specificity analyses, most sequenced polymyxin family BGCs are similarly predicted to produce NRP decapeptides that are either identical or nearly identical to previously characterized natural products (Fig. 1d). However, in one case, which we have called the mac BGC the predicted decapeptide differs from the consensus sequence by 4 amino acids, which is a larger difference than is seen in any previously reported congeners. Like colistin it contains a Leu instead of the more commonly seen Phe at position 6. In addition, at positions 3, 7 and 10, it contains a Ser, an Ile and a Leu instead of the 2, 4-diaminobutyric acid (Dab), Leu and Thr that are found in the consensus structure. As one of the strongest selective pressures for the creation of new congener structures is likely the development of resistance to previous antibiotics in a family, this divergent structure was of particular interest because of the chance it could represent the most evolved natural response to antibiotic resistance seen to date.
Although natural product isolation has traditionally relied on the analysis of bacterial fermentation broths, this process remains resource intensive and is limited by the fact that the majority of BGCs remain silent in laboratory-based fermentation studies.19 With the increasing accuracy of bioinformatic algorithms for predicting natural product structures, total chemical synthesis of the bioinformatically predicted BGC product (i.e., a synthetic bioinformatic natural product, syn-BNP) provides an alternate and potentially more straightforward method for accessing small molecules encoded by some sequenced BGCs.20–22 To access the predicted product of the mac BGC, we used solid-phase synthesis to generate its bioinformatically predicted linear decapeptide (Fig. 1e). This was then N-terminally acylated with (S)-6-methyloctanoic acid, which is the lipid most commonly seen in this family of antibiotics. Cyclization through the Dab at position 4 and deprotection gave a syn-BNP that we named macolacin (mcr-1 active colistin-like antibiotic) (Extended Fig. 1, Fig. S1–S2).
Antibacterial activity:
We initially assayed macolacin against the ESKAPE pathogens, which are commonly associated with antibiotic-resistant nosocomial infections (Table 1). Consistent with colistin and polymyxin B, macolacin showed potent, narrow spectrum Gram-negative activity macolacin and colistin are essentially equipotent against Klebsiella pneumoniae, A. baumannii, and macolacin is slightly less active than colistin against Pseudomonas aeruginosa and Enterobacter cloacae.
Table 1 |.
MIC values of macolacin, polymyxin B and colistin against a panel of sensitive and resistant ESKAPE pathogens (n=2). S=colistin sensitive (MIC ≤2 μg/mL), R=colistin resistant (MIC >2 μg/mL).
| Strain | S/R | Polymyxin | Colistin | Macolacin | |
|---|---|---|---|---|---|
|
| |||||
| ESKAPE pathogens | MIC μg/mL | ||||
| E. faecium Com15 | R | >128 | >128 | >128 | |
| S. aureus SH1000 | R | >128 | >128 | >128 | |
| K. pneumoniae 10031 | S | 1 | 0.5 | 1 | |
| A. baumannii 17978 | S | 0.5 | 1 | 1 | |
| P. aeruginosa PA01 | S | 2 | 1 | 4 | |
| E. cloacae 0150 | S | 1 | 1 | 4 | |
|
|
|
||||
| Clinical Isolates | |||||
| K. pneumoniae 0497 | R | mcr-1 | 32 | 32 | 2 |
| S. typhimurium 0635 | R | mcr-1 | 16 | 16 | 4 |
|
| |||||
| mcr-1 engineered pairs | |||||
| K. pneumoniae ATCC13883 | S | 1 | 1 | 1 | |
| K. pneumoniae ATCC13883 (pMQ124-mcr-1) | R | mcr-1 | 128 | 64 | 4 |
| A. baumannii ATCC17978 | S | 0.5 | 1 | 1 | |
| A. baumannii ATCC17978 (pMQ124xlab1-mcr-1) | R | mcr-1 | 16 | 32 | 2 |
| A. baumannii SM1536 | S | 1 | 0.5 | 2 | |
| A. baumannii SM1536 (pMQ124xlab1-mcr-1) | R | mcr-1 | 128 | 128 | 8 |
|
| |||||
| phoP/Q engineered pairs | |||||
| E. cloacae ATCC13047 (ΔphoP/Q) | R | 1 | 1 | 1 | |
| E. cloacae ATCC13047 (ΔphoP/Q+phoP/Q) | R | phoP/Q | 32 | 32 | 2 |
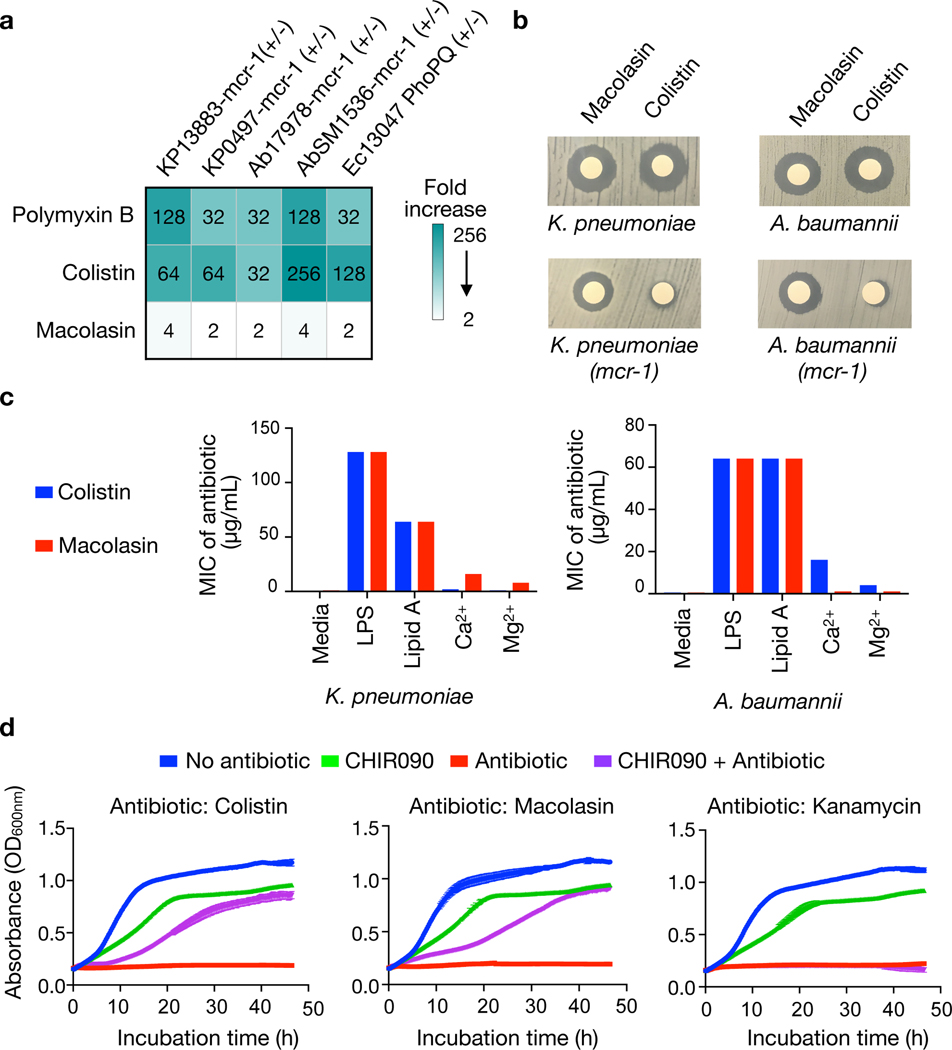
To specifically examine the activity of macolacin against PEtN transferase conferred colistin resistance, we used pairs of colistin sensitive and resistant strains of K. pneumoniae and A. baumannii that were generated by transformation with an mcr-1 containing plasmid (pMQ124-mcr-1 or pMQ124xlab1-mcr-1, respectively10). In the case of colistin and polymyxin B, mcr-1 expression led to a 32-fold or greater increase in MIC. However, macolacin showed only a 2 to 4-fold increase in MIC, even at the highest levels of colistin resistance (Table 1, Fig. 2a, b). Although macolacin’s activity largely mimics that of colistin and polymyxin B against colistin sensitive pathogens, it provides significantly improved activity against colistin resistant pathogens.
Fig. 2 |. Antibacterial activity of macolacin.
a) Fold increase in MIC for polymyxin, colistin and macolacin upon introduction of the mcr-1 resistance gene into K. pneumoniae or A. baumannii. b) Disc diffusion assays (10 μg of antibiotic/disk) against K. pneumoniae and A. baumannii with or without the mcr-1 resistance gene. c) MIC of colistin or macolacin against K. pneumoniae and A. baumannii (n=2) upon addition of different cell wall components to the culture media. d) Growth curves (n=3) for cultures of A. baumannii (blue) as well as A. baumannii in the presence of either the LpxC inhibitor CHIR-090 (green), one of three different antibiotics (colistin, macolacin or kanamycin) (red) or both CHIR-090 and an additional antibiotic (purple).
Another common lipid A modification that confers colistin resistance is the addition of 4-amino-4-deoxy-L-arabinose (L-Ara4N) to a phosphate on the lipid A.23 This intrinsic resistance mechanism is controlled by the activation of phoP/Q or pmrA/B, two-component regulators that control the expression of L-Ara4N transferase genes (e.g., arnT). When we compared MICs against E. cloacae in which phoP/Q was deleted to a strain where this deletion was rescued by transformation with a plasmid that expresses phoP/Q we observed a similar phenomenon to what we observed with mcr-1 resistance. The knockout strain was sensitive to polymyxin family antibiotics while the engineered strain was sensitive to macolacin but resistant to colistin as well as polymyxin B (Table 1).
Mode of action:
We reasoned that macolacin’s ability to overcome colistin resistance could result from either having a distinct mode of action or from its unique structure retaining the ability to interact with modified lipid A. The addition of either lipid A or lipopolysaccharide (LPS) to the assay media caused a significant increase in macolacin’s MIC (Fig. 2c). Although suppression of antibacterial activity in this assay is indicative of macolacin retaining the ability to interact with lipid A, it alone did not rule out the possibility of macolacin’s antibacterial activity arising from a different molecular target. In A. baumannii LPS is not essential and therefore lipid A biosynthesis inhibitors, like the LpxC inhibitor CHIR-09024, do not prevent A. baumannii growth in the laboratory.25–27Although CHIR-090 does not inhibit A. baumannii growth, its inhibition of lipid A biosynthesis prevents the production of LPS thereby rescuing A. baumannii from colistin toxicity. If macolacin’s antibiosis remains due to its interaction with lipid A, its activity should be similarly suppressed in the presence of CHIR-090. Although A. baumannii cultures treated with CHIR-090 grow more slowly than untreated cultures, they reach saturation after 48 hours (Fig. 2d). At concentrations above their MICs both macolacin and colistin completely abrogated A. baumannii growth over this same time period. The inclusion of CHIR-090 to cultures exposed to either macolacin or colistin rescued A. baumannii growth, indicating that macolacin not only retains the ability to bind lipid A but that its antibacterial activity likely remains the result of this interaction (Fig. 2d).
Structure activity relationship (SAR):
Macolacin differs from colistin by 3 amino acids. To determine which of these changes is critical for its ability to overcome mcr-1 encoded resistance we synthesized a set of structures with unique two amino acid changes (Extended Table 4). The change of Leu to Ile at position 7 (macolacin-7I) had little effect on mcr-1 encoded resistance. Individually, the changes at position 3 (Dab to Ser) or 10 (Thr to Leu) each appear to provide some protection against colistin resistance (MICs ≥ 8 μg/mL); however, potent antibacterial activity was only seen when both position 3 was serine and position 10 was leucine (MIC 1–2 μg/mL). The same pattern of activity was seen for phoP/Q regulated colistin resistance in E. cloacae. Although serine and leucine have individually been seen at these positions in characterized polymyxin congeners, these substitutions are rare compared to other observed amino acid changes (i.e., two cases for Ser and one case for Leu) and they have never appeared together at these positions in the same congener.
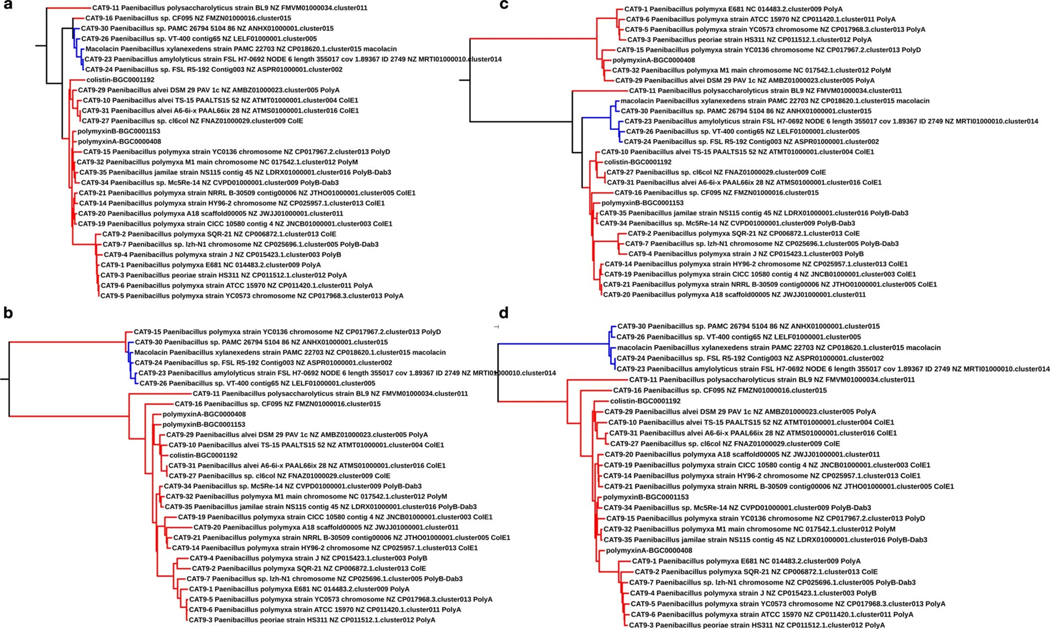
Phylogenetic analyses of gene sequences from polymyxin BGCs show a more significant divergence of mac gene sequences than is seen for genes from most other predicted polymyxin-family BGCs (Extended Fig. 2). This divergence undoubtedly long predates the recent global spread of mcr-1. Although the selection of the mac BGCs is likely in response to genome integrated PEtN transferase or intrinsic phoP/Q resistance genes; the fact that clinical and natural resistance arise from the same limited pool of potential resistance genes means this natural solution could prove useful for combating antibiotic resistant pathogens in the clinical setting.
Improved resistance activity:
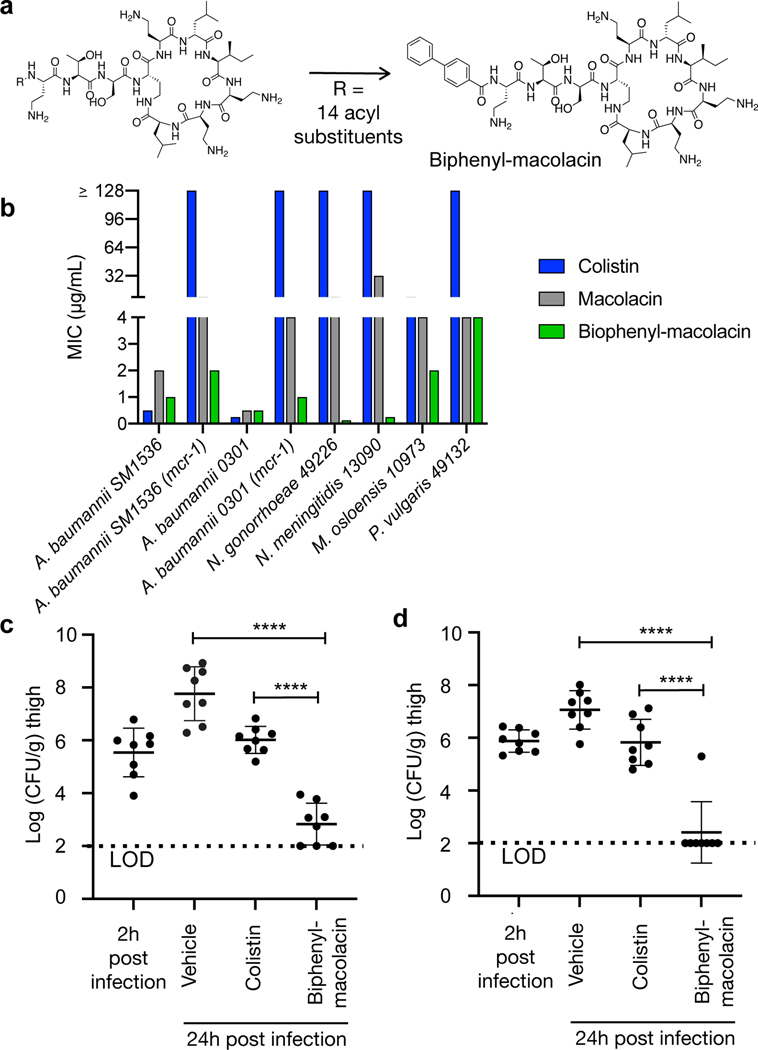
Among antibiotic resistant Gram-negative pathogens, CRAB (carbapenem-resistant A. baumannii) are classified as the highest level threat by the Center for Disease Control (CDC) in the United States.28 Colistin has emerged as a critical therapeutic option for the treatment of these pathogens. Unfortunately, strains containing mcr-1, or a related mcr gene, are increasingly found among clinical isolates around the world.29 We therefore chose to focus our translational efforts on developing a macolacin analog that would be effective at treating highly colistin resistant CRAB infections. Against A. baumannii that is resistant to ≤ 32 μg/mL of colistin, macolacin retains potent antibacterial activity (MIC 2 μg/mL). For extremely highly colistin resistant A. baumannii (MIC ≥128 μg/mL), macolacin’s MIC increased slightly to 8 μg/mL (Fig. 3b, Table 1). Although macolacin was significantly more potent than colistin, it’s MIC exceeds the threshold set by many global health agencies for clinical use. A very similar activity profile was seen for the intrinsically resistant N. gonorrhoeae.
Fig. 3 |. In vitro and in vivo activity of biphenyl-macolacin.
a) Among the macolacin analogs we synthesized with different lipid substituents, biphenyl-macolacin was the most potent analog against mcr-1 containing pathogens. b) MIC values of biphenyl-macolacin, macolacin and colistin against colistin sensitive and resistant pathogens (n=3 in duplicate). CFU counts from a neutropenic thigh infection model using A. bauamnnii-SM1536-mcr-1 (c) or A. baumannii-0301-mcr-1 (d). Two hours after mice were exposed to the pathogen, mice were subcutaneously given biphenyl-macolacin (20 mg/kg), colistin (20 mg/kg) or vehicle alone (0.9% saline) at 6 hour intervals. After 24 hours colony forming units (CFUs) were determined from homogenized thigh tissue samples. Significant differences between groups were analyzed by one-way analysis of variance (ANOVA) (****P<0.0001) (n=4 mice, n=8 thighs). Mean CFU counts and SD are shown.
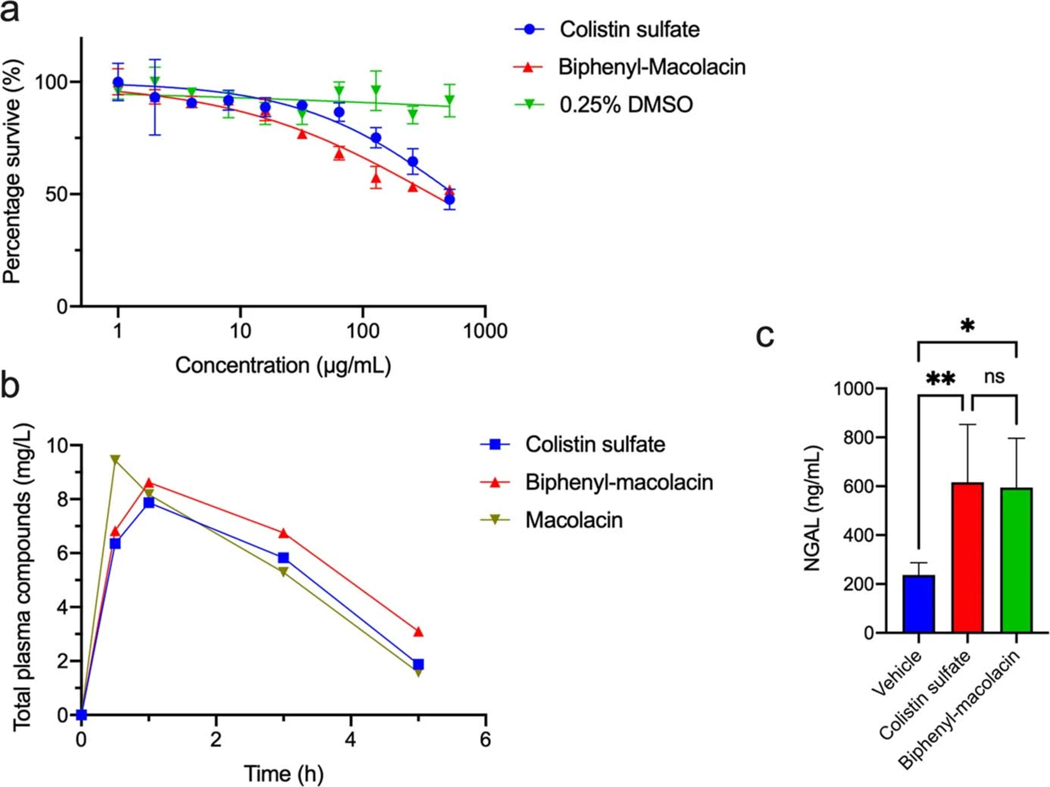
Before beginning animal studies with macolacin we sought to improve its activity against highly colistin resistant pathogens. As we believed the macolacin peptide macrocycle may have already been naturally optimized to interact with both modified and nonmodified lipid A head groups, we predicted that it would likely prove challenging to further improve the activity of macolacin through modifications of its peptide structure. The lipid, on the other hand, makes nonspecific hydrophobic interactions with the long acyl substituents of lipid A (Extended Fig. 3, Extended Table 5). Previous structure activity studies with polymyxins have found that the length of the lipid is important and that hydrophilic substituents on the lipid decrease antimicrobial activity.30,31 Within the relatively narrow parameters defined by these studies, we generated a collection of differentially N-acylated macolacin analogs to test for improved activity against highly resistant CRAB. In this study we identified a biphenyl lipid analog (biphenyl-macolacin) that is more active than macolacin against most of the pathogens we tested (Fig. 3a, b, Extended Table 2, Fig. S3–S4). Colistin and biphenyl-macolacin showed similarly low cytotoxicity to human cells (IC50 > 512 μg/mL, Extended Fig. 4a). Against CRAB, as well as a panel of XDR (extremely drug resistant) A. baumannii clinical isolates, biphenyl-macolacin had MICs of < 2 μg/mL even when these bacteria were transformed with mcr-1. Biphenyl-macolacin was also active against a number of intrinsically colistin resistant pathogens. In the case of N. gonorrhoeae, which is colistin resistant due to a chromosomally encoded PEtN transferase, biphenyl-macolacin was particularly potent (MIC 0.125 μg/mL). In the case of Proteus vulgaris, a common cause of urinary tract infections that is intrinsically colistin resistant due the modification of its LPS with L-Ara4N, biphenyl-macolacin had an MIC of 4 μg/mL, while colistin and polymyxin had MICs >128 μg/mL.
Animal studies:
A neutropenic thigh infection model was used to evaluate the efficacy of biphenyl-macolacin in vivo. In these studies we used two different highly colistin resistant strains of A. baumannii. One was a CRAB strain transformed with mcr-1 to give a colistin resistant strain (A. baumannii-SM1536-mcr-1). The second was an XDR A. baumannii clinical isolate (A. baumannii-0301) that was resistant to all antibiotics tested (Extended Table 3).32 When it was transformed with mcr-1 to give a PDR (pan drug resistant, XDR with colistin resistance) strain, colistin’s MIC increased to >64 μg/ml, but the activity of biphenyl-macolacin remained <2 μg/ml. For both studies two hours after mice were exposed to the pathogen, they were subcutaneously treated with antibiotic. The pharmacokinetic profiles of subcutaneous dosed colistin and biphenyl-macolacin indicated parity in plasma drug exposure for both compounds (Extended Fig. 4b). Colistin and biphenyl-macolacin also induced similar levels of neutrophil gelatinase associated lipocalin (NGAL), which is a biomarker for nephrotoxicity (Extended Fig. 4c).33–35 The bacterial burden in each infected thigh was determined after 24 hours. Colistin did not reduce the bacterial burden below the level of the initial infection. However, against both A. baumannii strains, biphenyl-macolacin showed significant antibacterial activity, resulting in an almost 5 log10 reduction in CFUs compared to the vehicle control group and a 3 log10 reduction in CFUs compared to the colistin treatment group (p < 0.0001 for both) (Fig. 3c, d).
Conclusion:
Colistin’s extensive use in livestock and human healthcare has resulted in the transfer of mcr-1 from the environment into the clinical setting thus threatening its use as an antibiotic of last resort against a number of MDR Gram-negative pathogens. As mcr-1-like PEtN transferase genes are common in the soil microbiome, we reasoned that natural selection might have led to colistin congeners that are capable of circumventing this troubling resistance mechanism. By coupling genome mining methods to identify polymyxin family-like BGCs in sequenced bacterial genomes with syn-BNP methods, we identified macolacin, a colistin-like antibiotic that is active against colistin resistant Gram-negative pathogens that is mediated by either mcr-1 or intrinsic transferase genes (eptA and arnT). Optimization of the lipid substituent in macolacin produced biphenyl-macolacin, which showed potent activity against Gram-negative bacteria that are resistant to colistin due to the acquisition of mcr-1 as well as against intrinsically colistin resistant pathogens (e.g., N. gonorrhoeae). Biphenyl-macolacin was active in vivo against both CRAB and XDR A. baumannii containing mcr-1, providing a new easily scaled therapeutic lead for this extremely troubling antibiotic resistant pathogen. Moving forward, additional animal model studies will be useful for identifying remaining issues that must be addressed to move biphenyl-macolacin through the development process. The systematic exploration of naturally occurring congeners of other antibiotics, whose utility is threatened by the rise of resistance in clinical settings, could prove similarly useful in uncovering activity against MDR pathogens.
Methods:
Identification and bioinformatic analysis of the macolacin (mac) biosynthetic gene cluster
A total of 36,957 nonribosomal peptide synthetase (NRPS) biosynthetic gene clusters (BGCs) from 10,858 bacterial genomes were downloaded from antiSMASH-db (version 2.0).36 The offline software package antiSMASH (v5.1.2, bacterial version)37 and BLAST were used to identify NRPS BGCs that resemble (by gene content, gene organization and sequence identity) BGCs known to encode polymyxin-family antibiotics (e.g., MIBIG IDs: BGC0000408, BGC0001192, BGC0001153). The linear NRP product of each predicted polymyxin/colistin-like BGC was determined using an A-domain substrate binding pocket analysis. In this analysis each NRPS A-domain in a predicted polymyxin/colistin-like BGC was analyzed using the online antiSMASH 5.0 (bacterial version) web tool to identify the 10-amino acids that make up its A-domain substrate binding pocket (i.e., amino acids 235, 236, 239, 278, 299, 301, 322, 330, 331, and 517). Each unknown A-domain substrate signature sequence was compared to a database of A-domain signatures from characterized BGCs to predict its amino acid substrate. The absence of post NRPS tailoring enzymes in polymyxin family BGCs means that the final linear peptide encoded by a BGC in this family can be predicted solely based on A-domain substrate specificity analysis. A domain sequences of polymyxin family BGCs were extracted using Geneious 11.1.5 software and aligned by MUSCLE algorithm using Macvector 18.0.2. The phylogenetic tree was visualized using online iTOL v6 software.
Peptide synthesis
Linear peptides were synthesized using standard solid phase Fmoc chemistry. Each peptide was synthesized using 2-Chlorotyityl resin preloaded with the first amino acid (0.2 g, 0.455 mmol/g). Resin was swollen in DCM for 30 min at room temperature and then washed with DMF (3 × 10.0 mL). The coupling proceeded by addition of the subsequent Fmoc protected amino acid (3.0 eq), 3.0 eq of DIPEA and 2.85 eq of HBTU in DMF (2.0 mL) to the resin. The resin was agitated under N2 for 60 min at room temperature and then washed with DMF (3 × 20.0 mL). The Fmoc protecting group was removed by addition of 20% piperidine in DMF (10.0 mL) to the resin with agitation under N2 for another 30 min. The resin was then washed with DMF (5 x 20.0 mL). The procedure of coupling and deprotection was repeated for each remaining amino acid. Fatty acids were activated and coupled to the N-termini of the resin bound linear peptide using the same procedure. The final linear lipo-peptide was cleaved from the resin by treatment with 10 mL of 1%TFA in DCM and stirring for 5 min. This was repeated twice and then air dried overnight. Cleaved linear lipo-peptide (0.2 mmol, 1 eq) was cyclized by dissolving in DCM (250.0 mL) containing TBTU (2.0 eq), HOBT (2.0 eq) and DIPEA (10 eq). After 1 h of stirring at room temperature, the mixture was washed twice with 1M HCl (100 mL) and then dried under vacuum. The dried crude cyclic lipo-peptide was treated with a cleavage cocktail (TFA: TIPS: H2O = 95%: 2.5%: 2.5%, 20.0 mL) for 1.5 h. Cold isopropyl ether was added to precipitate the TFA treated peptide and the precipitate was collected by centrifugation (3,000 x g, 5 min). Crude peptide pellets were dissolved in 5 mL methanol and then dried under vacuum overnight. Pure cyclic lipo-peptides were obtained by semipreparative HPLC.
Microbial susceptibility assay
Syn-BNPs were tested against the panel of Gram-positive and Gram-negative pathogens detailed in Table S1. MIC assays were conducted following the protocol recommended by the Clinical and Laboratory Standards Institute.38,39 Briefly, all assays were performed in duplicate (n=2) and at least two independent times in non-treated 96-well microliter plates (Thermo Scientific™ Nunc MicroWell 96-Well Microplates, Non-treated polystyrene plates). Syn-BNP peptides were dissolved in sterile DMSO (ATCC, USA) to give 12.8 mg/mL stock solutions. Polymyxin B sulfate (Sigma, USA) and colistin sulfate (Sigma, USA) were used as positive controls. Stock solutions were diluted across 96-well plates using a 2-fold serial dilution to give a concentration range of 256 to 0.25 μg/mL in 50 μL of LB broth per well. The top and bottom rows of each plate were filled with 100 μL of LB broth without compound to avoid edge effects. The last well in each row was treated as a negative control. It contained bacteria but no test compounds. A single colony of each assayed bacterial strain was inoculated into 5 mL of LB broth medium and grown overnight at 37 °C (200 rpm). For assays using bacteria containing mcr-1 expression plasmids, the appropriate selection antibiotic was added to keep the plasmid stable (concentrations are listed in Table S1). Saturated overnight cultures were diluted 5,000-fold in fresh LB, and then 50 μL was transferred into individual wells of a 96-well plate. Finally, each well contained a total volume of 100 μL to give a final assay concentration range of 128 to 0.125 μg/mL for each compound. MIC values were determined by visual inspection to identify the minimum concentration that completely prevented bacterial growth after 16 h at 37 °C.
CHIR-090 inhibition assay
To test microbial susceptibility of LPS-deficient bacteria to macolacin and colistin, the LPS inhibitor CHIR-090 was used to treat A. baumannii together with either macolacin, colistin or kanamycin.40 Colistin (Sigma, USA) and kanamycin (Sigma, USA) were used as positive and negative controls, respectively. A single colony of A. baumnanii ATCC17978 was inoculated into 5 mL of LB broth and grown overnight at 37 °C. Stationary-phase cultures were then diluted with fresh LB to an optical density (OD) at 600 nm of 0.2 and used as starter cultures for susceptibility assays. Assays were performed in triplicate in a 96-well plate. Assay wells contained 180 μL of starter culture bacteria, antibiotic at a final concentration of 10x its MIC and 10 μL of CHIR-090 (8 μg/mL, Sigma, USA). The final volume of each well was adjusted to 200 μL with LB. The Plate was covered with a clear lid and statically incubated at 37 °C in a Tecan plate reader (Infinite M Nano). The absorbance of each well was continuously measured at an absorbance wavelength of OD 600nm at every 15 min for 48 h. For each condition growth curves were run on three unique colonies (n=3). The growth curve at each concentration was plotted in Prism 9.0.
Pharmacokinetics assessment
All pharmacokinetic animal studies were ethically reviewed and carried out in accordance with the Institutional Animal Care and Use Committee of Hackensack Meridian Health under protocol number 269.01. The room was set on a twelve hour light cycle and the temperature was set to 70 °F. The humidity was set to 30%. Six-week old CD-1 female mice (20–25 g) were used in pharmacokinetic studies. Macolacin and biphenyl-macolacin were administered as a single dose by subcutaneous injection at 10 mg/kg using 0.9 % saline vehicle. Aliquots of 20 μL of blood were taken by puncture of the lateral tail vein from each mouse (n = 2 per route and dose) at 30 minutes, 1, 3, and 5 hours post-dose and captured in CB300 blood collection tubes containing K2EDTA and stored on ice. Plasma was recovered after centrifugation and stored at −80 °C until analyzed by high pressure liquid chromatography coupled to tandem mass spectrometry. 1 mg/mL stocks of macolacin and biphenyl-macolacin in water were serially diluted in 50% acetonitrile/water to generate standard curves and quality control spiking solutions. Standards and QCs were created by adding 10 μL of spiking solutions to 90 μL of drug free plasma (CD-1 K2EDTA Mouse, Bioreclamation IVT). 10 μL of control, standard, QC, or study samples were added to 10 μL of internal standard. Verapamil (Sigma Aldrich) was used as an internal standard for macolacin, and macolacin was used as an internal standard for biphenyl-macolacin. The compounds were extracted by adding 100 μL of 50% Methanol / 50% (10% Trichloroacetic acid in water) precipitation solvent. Extracts were vortexed for 5 minutes and centrifuged at 4,000 rpm for 5 minutes. 75 μL of supernatant was transferred for HPLC-MS/MS analysis and diluted with 75 μL of Milli-Q deionized water. LC-MS/MS analysis was performed on a Sciex Applied Biosystems Qtrap 6500+triple-quadrupole mass spectrometer coupled to a Shimadzu Nexera X 2 UHPLC system to quantify each drug in plasma. Chromatography was performed on a Phenomenex Luna Omega Polar C18 (2.1×100 mm; particle size, 3 μm) using a reversed phase gradient. Milli-Q deionized water with 0.1% formic acid was used for the aqueous mobile phase and 0.1% formic acid in acetonitrile for the organic mobile phase. Multiple-reaction monitoring of parent/daughter transitions in electrospray positive-ionization mode was used to quantify the analytes. The double charged ions were used for macolacin and biphenyl-macolacin. The following MRM transitions were used for maclamycin (585.15/240.90), maclamycin-L10 (604.91/152.00), and Verapamil (455.40/165.00). Sample analysis was accepted if the concentrations of the quality control samples were within 20% of the nominal concentration. Data processing was performed using Analyst software (version 1.6.2; Applied Biosystems Sciex).
Neutropenic thigh infection model
Six-week old female outbred Swiss Webster mice (20–25 g) were used for this experiment. Mice were randomly housed in individually ventilated cages (IVC) and maintained in accordance with American Association for Accreditation of Laboratory Care criteria. The room was set on a twelve hour light cycle and the temperature was set to 70 °F. The humidity was set to 30%. A. baumannii SM1536-(mcr-1) or A. baumannii-0301-(mcr-1) was grown in cation adjusted Mueller Hinton (MH) broth containing 50 μg/mL of gentamycin at 37 °C with shaking overnight. The cultures were centrifuged, supernatant aspirated and the bacteria were gently washed twice in sterile saline. The optical density was checked at 600 nm and diluted so that the bacterial suspension provided a challenge inoculum of approximately 1.0 x106 CFU per mouse thigh in a volume of 0.05 mL. Inoculum counts were verified by viable counts on Mueller Hinton Agar plates spread with proper dilutions of the inoculum and incubated at 37 °C for 24 h. Mice were rendered neutropenic by receiving 150 mg/kg and 100 mg/kg of cyclophosphamide via IP injection on day −4 and day −1 prior to infection, respectively. Mice were given 100 μL of vehicle (0.9% saline), colistin (20 mg/k) or biphenyl-macolacin (20 mg/k) at 2, 8, 14, & 20 hours post infection via subcutaneous injection. At 2 h post infection, mice in the untreated control infection group (n=4 mice/n=8 thighs) were humanely euthanized by CO2 narcosis to determine the starting thigh bacterial burden. All mice were closely monitored post-infection for morbidity. Any abnormal clinical signs were recorded if observed. Mice were humanely euthanized by CO2 narcosis at the experimental endpoint of 24 h post infection (n=4 mice/n=8 thighs for each condition). Thigh muscles were aseptically removed, weighed, homogenized and enumerated for bacterial burden by CFU counts after plating on MH agar containing 50 μg/mL of gentamicin. The treatment efficacy was determined both as the bacterial burden reduction in the thighs relative to the vehicle and colistin treated controls. All graphic data were statistically analyzed using computer Prism software (Prism 9). Burden differences between testing and control groups was assessed using one-way analysis of variance (ANOVA). A P value of < 0.05 was considered statistically significant. These animal studies were ethically reviewed and carried out in accordance with the Institutional Animal Care and Use Committees at the Hackensack Meridian Health’s under protocol 260 and the Rockefeller University under protocol 19032-H.
MTT cytotoxicity assay:
An MTT (3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay was used to determine cytotoxicity as previously described.41 In brief, HT29 cells cultured in DMEM (with 10% fetal bovine serum) were passaged in a 96- well plate with a density of 2,500 cells/well and cultured for 24 h at 37 with 5% CO2. Compounds with different concentrations were then added into each well. After 48 h of incubation, the media was removed and MTT solution (0.45 mg/mL) was added into each well. After 3 h of incubation, the solution was aspirated. Precipitated formazan crystals were dissolved by addition of 100 μL solubilization solution (40% DMF, 16% SDS and 2% acetic acid in H2O). The absorbance of each well was measured at OD570nm using a microplate reader (Epoch Microplate Spectrophotometer, BioTek). Taxol was used as the positive control. IC50 values were calculated by Prism 9.0 as the concentration of each compound required for 50% inhibition of cell growth compared to the no compound controls. All the experiments were performed in triplicates (n=3) and repeated two independent times.
Nephrotoxicity assay:
Six-week old female outbred Swiss Webster mice (20–25 g) were used in this experiment. The room was set on a twelve hour light cycle and the temperature is set to 70 °F. The humidity was set to 30%. After 3 days of acclimation, mice were randomly divided into 3 groups (n=6 in each group): vehicle group, colistin sulfate group and biphenyl-macolacin group. Mice were subcutaneous injected (SC) with 100 μL of 0.9 % saline (vehicle group), 20 mg/kg colistin sulfate or 20 mg/kg biphenyl-macolacin for 7 consecutive days. Serum was collected from blood samples 12 h after the last dose. The concentration of serum Neutrophil Gelatinase Associated Lipocalin (NGAL) was measured using a commercially available mouse NGAL ELISA Kit (R&D system, USA). The ELISA assay followed the manufacture’s instruction. The animal study was ethically reviewed and carried out in accordance with the Institutional Animal Care and Use Committees at the Rockefeller University under protocol 19032-H.
Extended Data
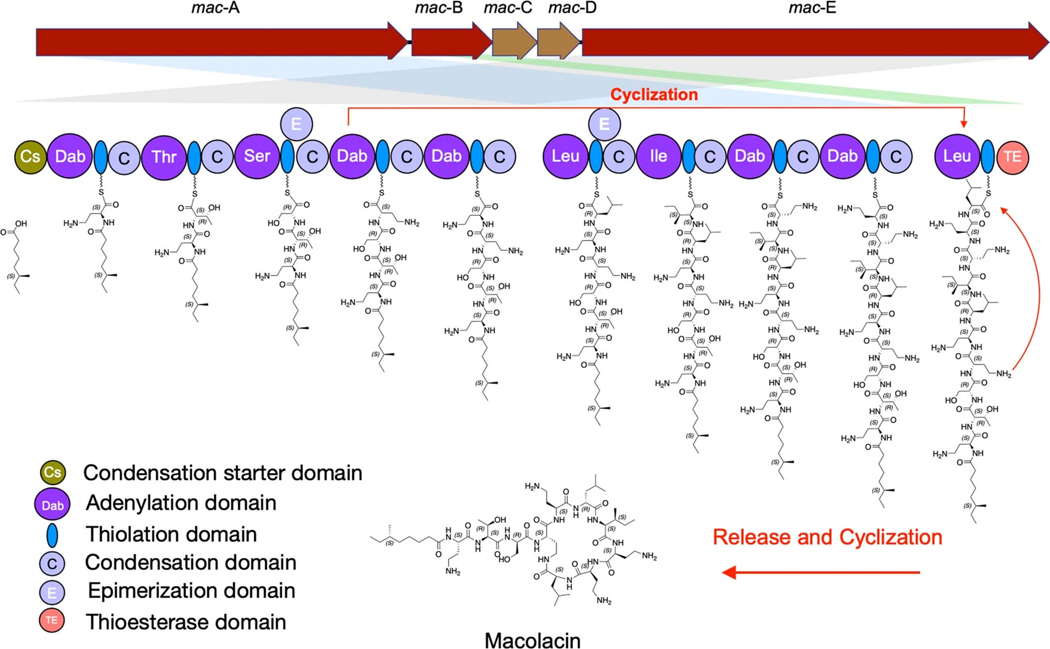
Extended Fig. 1 |. Proposed macolacin biosynthetic pathway.
The predicted biosynthetic scheme for macolacin based on detailed bioinformatic analysis of the mac BGC is depicted.
Extended Fig. 2 |. Phylogenetic trees constructed from A-domain sequences associated with complete colistin and macolacin BGC.
Phylogenetic trees constructed from A domain sequences associated with complete colistin and macolacin A BGC. a) A1 domain; b) A3 domain; c) A7 domain and d) A10 domain. Each A-domain sequence was extracted from the polymyxin-like BGCs was then aligned together with known characterized polymyxin BGCs (e.g., MIBIG IDs: BGC0000408, BGC0001192, BGC0001153) using the MUSCLE alignment software. The resulting phylogenetic tree was visualized using iTOLv5 software. Red color represents hits in polymyxin clade. Blue color represents hits in macolacin clade.
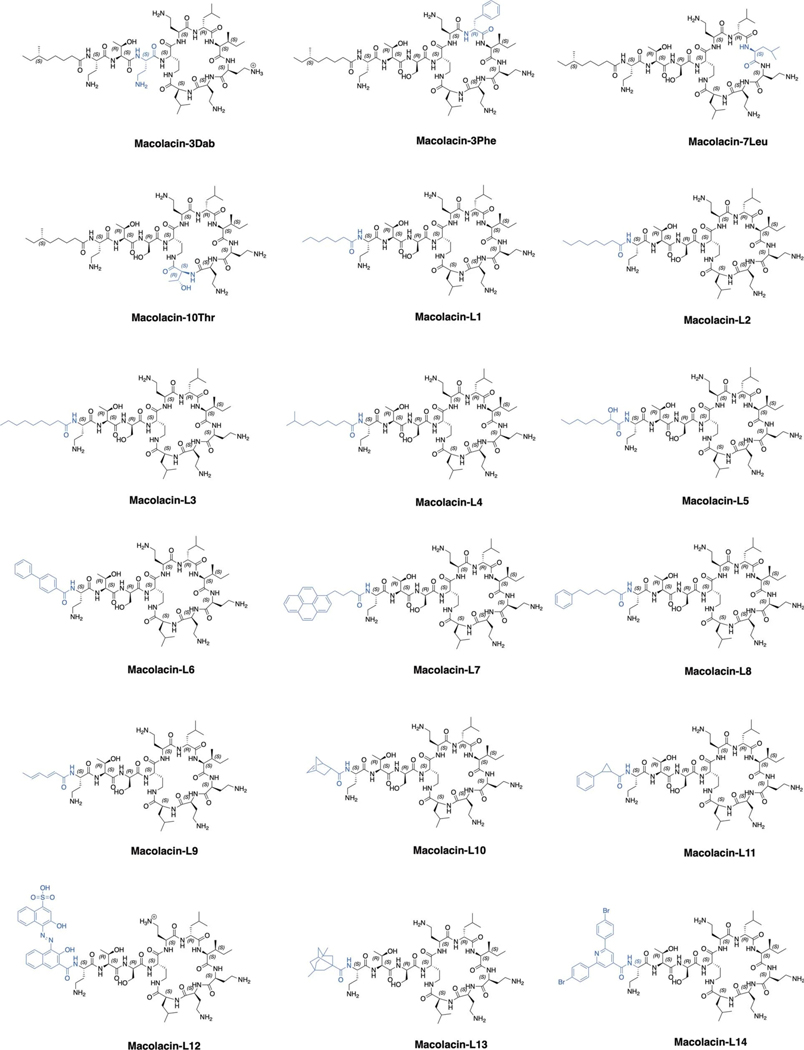
Extended Fig. 3 |. Structures of all synthetic macolacin derivates.
Structural differences compared to macolacin are depicted in blue.
Extended Fig. 4 |. Cytotoxicity and pharmacokinetic evaluation of macolacin and biphenyl-macolacin.
a) Cytotoxicity of macolacin and biphenyl-macolacin against HEK293. Data are presented as means ± SD. n=3 technical replicates. b) Pharmacokinetic assessment of macolacin and biphenyl-macolacin. Total plasma concentrations of macolacin, biphenyl-macolacin or colistin versus time after administration of a single subcutaneous dose (10 mg/kg) to neutropenic mice. n=2 biologically independent mice. Data are presented as mean of two independent assays. c) The level of serum NGAL in colistin or biphenyl-macolacin treated mice. Significant differences between groups were determined by one-way analysis of variance (ANOVA) (*P<0.05) (n=6 biologically independent mice). Data are presented as means ± SD. Vehicle vs. Colistin, P value=0.0069; Vehicle vs. Biphenyl-macolacin, P value=0.0104; Colistin vs. Biphenyl-macolacin, P value=0.9773.
Extended Table 1 |. Macolacin A-domain specificity analysis.
Note: Red text (I) indicates a difference between the signature code of macolacin and the closest known signature from the BGC of a characterized natural product.
| Macolacin A-domains | Closest characterized A-domain | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | 235 | 236 | 239 | 278 | 299 | 301 | 322 | 330 | 331 | 517 | Substrate prediction | 235 | 236 | 239 | 278 | 299 | 301 | 322 | 330 | 331 | 517 | Source |
| A1 | D | V | G | E | I | S | S | I | D | K | Dab | D | V | G | E | I | S | S | I | D | K | Polymyxin B |
| A2 | D | F | W | N | I | G | M | V | H | K | Thr | D | F | W | N | I | G | M | V | H | K | Polymyxin B |
| A3 | D | V | W | H | F | S | L | V | D | K | Ser | D | V | W | H | F | S | L | V | D | K | Polymyxin B |
| A4 | D | V | G | E | I | S | S | I | D | K | Dab | D | V | G | E | I | S | S | I | D | K | Polymyxin B |
| A5 | D | V | G | E | I | s | S | I | D | K | Dab | D | V | G | E | I | S | S | I | D | K | Polymyxin B |
| A6 | D | A | W | I | V | G | A | I | V | K | Leu | D | A | W | I | V | G | A | I | V | K | Polymyxin B |
| A7 | D | G | F | F | L | G | V | I | F | K | lie | D | G | F | F | L | G | V | V | F | K | Bacitracin |
| A8 | D | V | G | E | I | S | S | I | D | K | Dab | D | V | G | E | I | S | S | I | D | K | Polymyxin B |
| A9 | D | V | G | E | I | S | S | I | D | K | Dab | D | V | G | E | I | S | S | I | D | K | Polymyxin B |
| A10 | D | A | W | I | V | G | A | I | V | K | Leu | D | A | W | I | V | G | A | I | V | K | Polymyxin B |
Extended Table 2 |. MIC values for macolacin analogs with different lipid substituents.
Note: For pathogens that are not intrinsically resistant to colistin, the fold increase in MIC upon introduction of mcr-1 is shown in bold. L1-L14 are the macolacin analogs shown in Extended Fig. 3. L6 is biphenyl-macolacin. S = colistin sensitive, R = colistin resistant, Col = colistin, Mac = macolacin. Each MIC was measured in technical duplicate and on at least two independent occasions with the same results.
| S/R | Col | Mac | L1 | L2 | L3 | L4 | L5 | L6 | L7 | L8 | L9 | L10 | L11 | L12 | L13 | L14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Pathogen | MIC ug/mL | ||||||||||||||||
|
| |||||||||||||||||
| A. bau mann ii SM1536 | S | 0.5 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 8 | 32 | 4 | 2 | 1 | 2 |
| A. baumannii SM 1536 (mcr-1) | R | 128 | 8 | 16 | 8 | 8 | 8 | 8 | 2 | 4 | 16 | 64 | 64 | 16 | 8 | 16 | 4 |
| Fold increase in MIC | 256 | 4 | 8 | 8 | 4 | 8 | 4 | 2 | 2 | 8 | 8 | 2 | 4 | 4 | 16 | 2 | |
|
| |||||||||||||||||
| A. baumannii 0301 | S | 0.25 | 0.5 | 2 | 2 | 2 | 2 | 2 | 0.5 | 4 | 4 | 4 | 16 | 1 | 2 | 0.5 | 2 |
| A. baumannii 0301 (mcr-1) | R | >128 | 4 | 4 | 4 | 4 | 2 | 4 | 1 | 4 | 4 | 8 | 16 | 4 | 2 | 2 | 2 |
| Fold increase in MIC | >512 | 8 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 4 | 1 | 4 | 1 | |
|
| |||||||||||||||||
| eptA intrinsic resistance | |||||||||||||||||
| N. gonorrhoeae 49226 | R | >128 | 8 | 64 | 32 | 8 | 2 | 4 | 0.125 | > 1 | 32 | >64 | >64 | 64 | 8 | 8 | 1 |
| N. meningitidis 13090 | R | 128 | 32 | >64 | >64 | 32 | 16 | 16 | 0.25 | 2 | 32 | >64 | >64 | 64 | 16 | 32 | 1 |
| M. osloensis 10973 | R | 8 | 4 | 8 | 4 | 16 | 8 | 8 | 2 | 8 | 2 | 16 | 32 | 4 | 4 | 4 | 4 |
|
| |||||||||||||||||
| amT intrinsic resistance | |||||||||||||||||
| P. vulgaris 49132 | R | >128 | 4 | 4 | 4 | 4 | 4 | 2 | 4 | 8 | 2 | >64 | >64 | 8 | >64 | 8 | 16 |
Extended Table 3 |. MIC data for XDR A. baumannii with and without mcr-1.
Note: MIC data below the dotted line was obtained from CDC & FDA Antibiotic Resistant Isolate Bank. Each MIC was measured in technical duplicate and on three independent occasions with the same results. S = colistin sensitive, R = colistin resistant.
| A. baumannii strain | 0287 | 0287 | 0295 | 0295 | 0301 | 0301 | 0286 | 0282 | 0296 |
| mcr-1 genotype | − | + | − | + | − | + | − | − | − |
| Colistin Resistance | S | R | S | R | S | R | S | S | S |
|
| |||||||||
|
Antibiotic MIC (μg/ml)
|
|||||||||
| Macolacin | 1 | 1 | 0.5 | 4 | 0.5 | 4 | 2 | 1 | 1 |
| Biphenyl-macolacin | 1 | 1 | 0.5 | 2 | 0.5 | 1 | 2 | 1 | 1 |
| Colistin | 0.5 | 64 | 0.25 | >128 | 0.25 | >128 | 1 | 0.5 | 0.25 |
|
| |||||||||
| Amikacin | 32 | 4 | 64 | >64 | >64 | >64 | |||
| Ampicillin | >32 | >32 | >32 | 32 | >32 | >32 | |||
| Cefepime | 32 | >32 | >32 | 32 | >32 | >32 | |||
| Cefotaxime | >64 | >64 | >64 | >64 | >64 | >64 | |||
| Ceftazidime | 128 | >128 | 128 | 128 | >128 | >128 | |||
| Ceftriaxone | >32 | >32 | >32 | >32 | >32 | >32 | |||
| Ciprofloxacin | >8 | >8 | >8 | >8 | >8 | >8 | |||
| Doripenem | >8 | >8 | >8 | >8 | >8 | >8 | |||
| Gentamicin | 4 | 4 | 8 | >16 | >16 | >16 | |||
| Imipenem | 64 | >64 | >64 | >64 | 64 | 64 | |||
| Imipenem/relebactam | >16 | >16 | >16 | >16 | >16 | >16 | |||
| Imipenem + chelators | >32 | >32 | >32 | >32 | >32 | >32 | |||
| Levofloxacin | 8 | >8 | >8 | 8 | 8 | >8 | |||
| Meropenem | >8 | >8 | >8 | >8 | >8 | >8 | |||
| Minocycline | <=4 | 16 | 16 | 8 | 8 | <=4 | |||
| Piperacillin | >128 | >128 | >128 | >128 | >128 | >128 | |||
| Tetracycline | >32 | >32 | >32 | >32 | >32 | 32 | |||
| Tigecycline | <=0.5 | 4 | 4 | 1 | 1 | 2 | |||
| Tobramycin | >16 | 1 | >16 | >16 | >16 | >16 | |||
| Trimethoprim | >8 | >8 | >8 | >8 | >8 | >8 | |||
Extended Table 4 |. SAR of amino acid differences between macolacin and colistin.
Note: Macolacin differs from colistin by three amino acids: Ser3, Ile7, Leu10 (orange). Macolacin analogs synthesized with only 2 amino acid changes (orange) compared to colistin were tested for antibacterial activity (MIC μg/mL) against pathogens with or without either mcr-1 or phoP/Q (n=2). The fold increase in MIC upon introduction of either mcr-1 or phoP/Q is shown in bold. In polymyxin family structures, the side chain of a D-configured amino acid at position 3 and an L-configured amino acid at position 10 are very close in three-dimensional space and likely interact together with lipid A to counter common amine containing modifications (i.e., PEtN or L-Ara4N). As the binding of colistin to lipid A is largely driven by electrostatic interactions, it is not surprising that compensating for appending a primary amine onto a lipid A phosphate involves replacing a positively charged Dab with a neutral residue (Ser).
| Substitution | Polymyxin | Colistin | Macolacin | Macolacin-3Dab | Macolacin-7Leu | Macolacin-10Thr |
|---|---|---|---|---|---|---|
| Amino acid 3 | Dab | Dab | Ser | Dab | Ser | Ser |
| Amino acid 7 | Leu | Leu | lie | lie | Leu | lie |
| Amino acid 10 | Thr | Thr | Let | Leu | Leu | Thr |
|
| ||||||
| Pathogen | MIC (μg/mL) | |||||
|
| ||||||
| K. pneumoniae 10031 | 1 | 0.5 | 1 | 1 | 1 | 0.5–1 |
| K. pneumoniae 0497 (mcr-1) | 32 | 16 | 2 | 8 | 2 | 8 |
|
| ||||||
| K. pneumoniae 13883 | 1 | 1 | 1 | 2 | 2 | 1 |
| K. pneumoniae 13883 (mcr-1) | 128 | 64 | 4 | 16 | 4 | 64 |
|
|
||||||
| Fold increase in MIC | 128 | 64 | 4 | 8 | 2 | 64 |
|
| ||||||
| E. cloacae ATCC13047 (Δ phoP/Q) | 1 | 1 | 1 | 2 | 2 | 1 |
| E. cloacae ATCC13047 (Δ phoP/Q+phoP/Q) | 32 | 32 | 2 | 8 | 1 | 16 |
|
|
||||||
| Fold increase in MIC | 32 | 32 | 2 | 4 | 0.5 | 16 |
Extended Table 5 |. High-resolution mass spectrometry data for all syn-BNP peptides.
Note: all HRMS data were collected in positive ionization model with a mass range from m/z 200–2000.
| Syn-BNPs | Molecular formular [M] | Theoretical [M+H]+ |
Observed [M+H]* | Mass error |
|---|---|---|---|---|
|
| ||||
| macolacin | C54H101N15O13 | 1168.7776 | 1168.7787 | 0.9 ppm |
| macolacin-3Dab | C55H104N16O12 | 1181.8092 | 1181.8074 | 1.5 ppm |
| macolacin-7Leu | C54H101N15O13 | 1168.7776 | 1168.7759 | 1.4 ppm |
| macolacin-10Thr | C52H98N15O13 | 1156.7412 | 1156.7379 | 2.8 ppm |
| macolacin-L1 | C52H97N15O13 | 1140.7463 | 1140.7424 | 3.4 ppm |
| macolacin-L2 | C53H99N15O13 | 1154.7620 | 1154.7566 | 4.6 ppm |
| macolacin-L3 | C54H101N15O13 | 1168.7776 | 1168.7751 | 2.1 ppm |
| macolacin-L4 | C55H103N15O13 | 1182.7933 | 1182.7882 | 4.3 ppm |
| macolacin-L5 | C53H99N15O14 | 1170.7569 | 1170.7531 | 3.2 ppm |
| macolacin-L6 | C58H93N15O13 | 1208.7150 | 1208.7091 | 4.8 ppm |
| macolacin-L7 | C65H99N15O13 | 1298.7620 | 1298.7574 | 3.5 ppm |
| macolacin-L8 | C54H101N15O13 | 1202.7620 | 1202.7547 | 6.0 ppm |
| Macolacin-L9 | C51H91N15O13 | 1122.6994 | 1122.6926 | -6.0 ppm |
| Macolacin-L10 | C53H93N15O13 | 1148.7150 | 1148.7120 | −2.6 ppm |
| Macolacin-L11 | C55H93N15O13 | 1172.7150 | 1172.7184 | 2.9 ppm |
| Macolacin-L12 | C66H97N17O18S | 1448.6991 | 1448.7029 | 2.6 ppm |
| Macolacin-L13 | C58H103N15O13 | 1218.7933 | 1218.7906 | −2.2 ppm |
| Macolacin-L14 | C63H94Br2N16 O13 | 1441.5626 | 1441.5622 | −0.3 ppm |
Supplementary Material
Acknowledgments
We thank Yohei Doi (pMQ124-mcr-1 and pMQ124xlab-mcr-1) and Joseph M. Boll (E. cloacae 13047-ΔphoP/Q and E. cloacae13047-ΔphoP/Q+phoP/Q) laboratories for providing strains and plasmids. We thank the Comparative Bioscience Center at the Rockefeller University for their help with the animal studies. This work was supported by the National Institutes of Health (1U19AI142731 and 5R35GM122559).
Abbreviations
- syn-BNP
synthetic bioinformatic natural product
- CRAB
carbapenem-resistant Acinetobacter baumannii
- NRPS
nonribosomal peptide synthetase
- BGC
biosynthetic gene cluster
- LPS
lipopolysaccharides
- PEtN
phosphoethanolamine
- L-Ara4N
4-amino-4-deoxy-L-arabinose
- MDR
multi-drug resistant
- XDR
extremely drug resistant
- PDR
pan drug resistant
- NGAL
neutrophil gelatinase associated lipocalin
- CFU
colony forming units
- MIC
Minimum inhibitor concentration
Footnotes
Data Availability Statement
Publicly available DNA sequence data used in this study are referenced accordingly. The macolacin BGC sequence is available in GenBank with accession number NZ_CP018620.1.cluster015. Other accession numbers for polymyxin-like BGCs appear in the Supplementary Table S2. NMR spectra for macolacin and diphenyl-macolacin are presented as Supplementary Information. BGCs were collected from antiSMASH-db (version 2.0). The website can be accessed through https://antismash-db.secondarymetabolites.org/. The raw data of Figure 2b, 2c, 3c, 3d and Extended Figure 4b, 4c can be found in the source data file for this manuscript.
Manuscript References:
- 1.Ventola CL The antibiotic resistance crisis: part 1: causes and threats. P T 40, 277–283 (2015). [PMC free article] [PubMed] [Google Scholar]
- 2.Payne DJ, Gwynn MN, Holmes DJ & Pompliano DL Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6, 29–40, doi: 10.1038/nrd2201 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Deveson Lucas D. et al. Emergence of High-Level Colistin Resistance in an Acinetobacter baumannii Clinical Isolate Mediated by Inactivation of the Global Regulator H-NS. Antimicrob Agents Chemother 62, doi: 10.1128/AAC.02442-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aitolo GL, Adeyemi OS, Afolabi BL & Owolabi AO Neisseria gonorrhoeae Antimicrobial Resistance: Past to Present to Future. Curr Microbiol 78, 867–878, doi: 10.1007/s00284-021-02353-8 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Tacconelli E. et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18, 318–327, doi: 10.1016/S1473-3099(17)30753-3 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Imai Y. et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 576, 459–464, doi: 10.1038/s41586-019-1791-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M. & Rolain JM Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti-Infe 10, 917–934, doi: 10.1586/Eri.12.78 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Liu YY et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infectious Diseases 16, 161–168, doi: 10.1016/S1473-3099(15)00424-7 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Jeannot K, Bolard A. & Plesiat P. Resistance to polymyxins in Gram-negative organisms. Int J Antimicrob Agents 49, 526–535, doi: 10.1016/j.ijantimicag.2016.11.029 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Liu YY et al. Structural Modification of Lipopolysaccharide Conferred by mcr-1 in Gram-Negative ESKAPE Pathogens. Antimicrob Agents Ch 61, doi:ARTNe00580-1710.1128/AAC.00580-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz S. & Johnson AP Transferable resistance to colistin: a new but old threat. J Antimicrob Chemother 71, 2066–2070, doi: 10.1093/jac/dkw274 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Hameed F. et al. Plasmid-mediated mcr-1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: first report from Pakistan. Rev Soc Bras Med Trop 52, e20190237, doi: 10.1590/0037-8682-0237-2019 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Tian GB et al. MCR-1-producing Klebsiella pneumoniae outbreak in China. Lancet Infect Dis 17, 577, doi: 10.1016/S1473-3099(17)30266-9 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Rutledge PJ & Challis GL Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat Rev Microbiol 13, 509–523, doi: 10.1038/nrmicro3496 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Sussmuth RD & Mainz A. Nonribosomal Peptide Synthesis-Principles and Prospects. Angew Chem Int Ed Engl 56, 3770–3821, doi: 10.1002/anie.201609079 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Stachelhaus T, Mootz HD & Marahiel MA The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chemistry & biology 6, 493–505 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Rabanal F. & Cajal Y. Recent advances and perspectives in the design and development of polymyxins. Nat Prod Rep 34, 886–908, doi: 10.1039/c7np00023e (2017). [DOI] [PubMed] [Google Scholar]
- 18.Li J, Nation R. & Kaye K. Polymyxin Antibiotics: From Laboratory Bench to Bedside Preface. Polymyxin Antibiotics: From Laboratory Bench To Bedside 1145, V–VI (2019). [Google Scholar]
- 19.Tomm HA, Ucciferri L. & Ross AC Advances in microbial culturing conditions to activate silent biosynthetic gene clusters for novel metabolite production. J Ind Microbiol Biotechnol 46, 1381–1400, doi: 10.1007/s10295-019-02198-y (2019). [DOI] [PubMed] [Google Scholar]
- 20.Chu J. et al. Discovery of MRSA active antibiotics using primary sequence from the human microbiome. Nat Chem Biol 12, 1004–1006, doi: 10.1038/nchembio.2207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu J, Vila-Farres X. & Brady SF Bioactive Synthetic-Bioinformatic Natural Product Cyclic Peptides Inspired by Nonribosomal Peptide Synthetase Gene Clusters from the Human Microbiome. J Am Chem Soc 141, 15737–15741, doi: 10.1021/jacs.9b07317 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu J. et al. Synthetic-Bioinformatic Natural Product Antibiotics with Diverse Modes of Action. J Am Chem Soc 142, 14158–14168, doi: 10.1021/jacs.0c04376 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang KN et al. Colistin heteroresistance in Enterobacter cloacae is regulated by PhoPQ-dependent 4-amino-4-deoxy-l-arabinose addition to lipid A. Mol Microbiol 111, 1604–1616, doi: 10.1111/mmi.14240 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClerren AL et al. A slow, tight-binding inhibitor of the zinc-dependent deacetylase LpxC of lipid A biosynthesis with antibiotic activity comparable to ciprofloxacin. Biochemistry 44, 16574–16583, doi: 10.1021/bi0518186 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffatt JH et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54, 4971–4977, doi: 10.1128/AAC.00834-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei J-R et al. LpxK is essential for growth of Acinetobacter baumannii ATCC 19606: relationship to toxic accumulation of lipid A pathway intermediates. MSphere 2, e00199–00117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richie DL et al. Toxic Accumulation of LPS Pathway Intermediates Underlies the Requirement of LpxH for Growth of Acinetobacter baumannii ATCC 19606. PLoS One 11, e0160918, doi: 10.1371/journal.pone.0160918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services, C (ed Department of Health and Human Services; ) (2019). [DOI] [PubMed] [Google Scholar]
- 29.Ling Z. et al. Epidemiology of mobile colistin resistance genes mcr-1 to mcr-9. J Antimicrob Chemother 75, 3087–3095, doi: 10.1093/jac/dkaa205 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Sakura N. et al. The contribution of the N-terminal structure of polymyxin B peptides to antimicrobial and lipopolysaccharide binding activity. Bulletin of the Chemical Society of Japan 77, 1915–1924, doi: 10.1246/bcsj.77.1915 (2004). [DOI] [Google Scholar]
- 31.Tsubery H, Ofek I, Cohen S. & Fridkin M. N-terminal modifications of Polymyxin B nonapeptide and their effect on antibacterial activity. Peptides 22, 1675–1681, doi: 10.1016/S0196-9781(01)00503-4 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Lutgring JD et al. FDA-CDC Antimicrobial Resistance Isolate Bank: a Publicly Available Resource To Support Research, Development, and Regulatory Requirements. J Clin Microbiol 56, doi: 10.1128/JCM.01415-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl 241, 89–94, doi: 10.1080/00365510802150158 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Ishfaq M, Fan Q, Chen C. & Li J. 7-Hydroxycoumarin Attenuates Colistin-Induced Kidney Injury in Mice Through the Decreased Level of Histone Deacetylase 1 and the Activation of Nrf2 Signaling Pathway. Front Pharmacol 11, 1146, doi: 10.3389/fphar.2020.01146 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolignano D. et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis 52, 595–605, doi: 10.1053/j.ajkd.2008.01.020 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Blin K. et al. The antiSMASH database version 2: a comprehensive resource on secondary metabolite biosynthetic gene clusters. Nucleic Acids Res 47, D625–D630, doi: 10.1093/nar/gky1060 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blin K. et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res 47, W81–W87, doi: 10.1093/nar/gkz310 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Testing E. C. o. A. S. Recommendations for MIC determination of colistin (polymyxin E) as recommended by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group. EUCAST: Växjö, Sweden (2016). [Google Scholar]
- 39.Wikler MA Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. CLSI (NCCLS) 26, M7–A7 (2006). [Google Scholar]
- 40.Bojkovic J. et al. Characterization of an Acinetobacter baumannii lptD Deletion Strain: Permeability Defects and Response to Inhibition of Lipopolysaccharide and Fatty Acid Biosynthesis. J Bacteriol 198, 731–741, doi: 10.1128/JB.00639-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmichael J, DeGraff WG, Gazdar AF, Minna JD & Mitchell JB Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 47, 936–942 (1987). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available DNA sequence data used in this study are referenced accordingly. The macolacin BGC sequence is available in GenBank with accession number NZ_CP018620.1.cluster015. Other accession numbers for polymyxin-like BGCs appear in the Supplementary Table S2. NMR spectra for macolacin and diphenyl-macolacin are presented as Supplementary Information. BGCs were collected from antiSMASH-db (version 2.0). The website can be accessed through https://antismash-db.secondarymetabolites.org/. The raw data of Figure 2b, 2c, 3c, 3d and Extended Figure 4b, 4c can be found in the source data file for this manuscript.