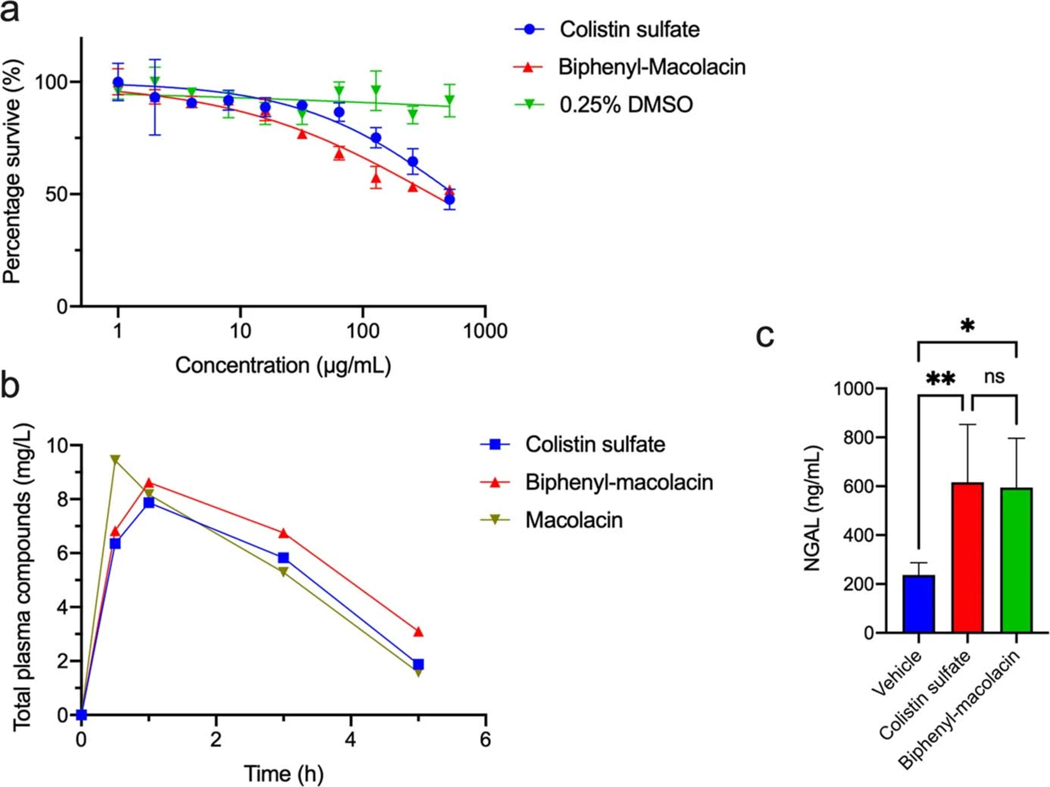
Extended Fig. 4 |. Cytotoxicity and pharmacokinetic evaluation of macolacin and biphenyl-macolacin.
a) Cytotoxicity of macolacin and biphenyl-macolacin against HEK293. Data are presented as means ± SD. n=3 technical replicates. b) Pharmacokinetic assessment of macolacin and biphenyl-macolacin. Total plasma concentrations of macolacin, biphenyl-macolacin or colistin versus time after administration of a single subcutaneous dose (10 mg/kg) to neutropenic mice. n=2 biologically independent mice. Data are presented as mean of two independent assays. c) The level of serum NGAL in colistin or biphenyl-macolacin treated mice. Significant differences between groups were determined by one-way analysis of variance (ANOVA) (*P<0.05) (n=6 biologically independent mice). Data are presented as means ± SD. Vehicle vs. Colistin, P value=0.0069; Vehicle vs. Biphenyl-macolacin, P value=0.0104; Colistin vs. Biphenyl-macolacin, P value=0.9773.