Abstract
The core function of the testes is to produce sperms, which is the prerequisite for maintaining male fertility. PIWI-interacting RNAs (piRNAs) are a class of non-coding small RNAs that are mainly enriched in the reproductive organ and play a key role in germ cell development and spermatogenesis. However, the expression and function of piRNAs in the testes of Tibetan sheep, a domestic animal endemic to the Tibetan Plateau, remain unknown. In this study, we evaluated the sequence structure, expression profile, and potential function of piRNAs in testicular tissues from Tibetan sheep at different developmental stages (3 months, 1 year, and 3 years of age, respectively) by small RNA sequencing. Of the identified piRNAs, the sequence lengths of 24–26 nt and 29 nt dominate. Most piRNA sequences begin with uracil and have a distinct ping-pong structure which mainly distributes in exons, repeat regions, introns, and other unannotated regions of the genome. The piRNAs in the repeat region are primarily derived from the retrotransposons: long terminal repeats, long interspersed nuclear elements, and short interspersed elements. These piRNAs constitute 2,568 piRNA clusters, which mainly distribute on chromosomes 1, 2, 3, 5, 11, 13, 14, and 24, and of these clusters, a total of 529 piRNA clusters were differentially expressed in at least two age groups. Most of the piRNAs were expressed in a low abundance in the testes of developing Tibetan sheep. A total of 41,552 and 2,529 differential piRNAs were identified in testes from 3 months vs. 1 year, and 1 year vs. 3 years, respectively, presenting significantly increased abundance for most piRNAs in 1 year and 3 years compared with 3 months. The functional evaluation of the target genes showed that the differential piRNAs are mainly involved in regulating gene expression, transcription, protein modification, and cell development during spermatogenesis and testicular development. In conclusion, this study focused on the sequence structure and expression characteristics of piRNAs in the testis of Tibetan sheep and provided new insights into the functional mechanism of piRNAs in testicular development and spermatogenesis of sheep.
Keywords: piRNA, piRNA cluster, spermatogenesis, Tibetan sheep, testis
This study unraveled the unique sets of piRNAs and their expression profiles for the first time, as well as potential functions in developmental Tibetan sheep. These findings broaden our knowledge on the complexity of transcriptional regulation during Tibetan sheep spermatogenesis, also providing novel and valuable information for further investigating the mechanism of spermatogenesis in sheep.
Introduction
Small RNAs in animals mainly include microRNAs (miRNAs), small interfering RNAs (siRNAs), and piwi-interacting RNAs (piRNAs) (Yang et al., 2019). Small RNAs often play an important role in gene silencing and epigenetic regulation by binding and directing AGO proteins to their specific targets (Liu et al., 2004; Meister, 2013). The AGO proteins have two subfamilies, namely, the somatic AGO and the germline-specific PIWI subfamilies (Thomson and Lin, 2009). miRNAs and siRNAs usually bind to proteins of the AGO subfamily ubiquitously occurring in animal tissues, and piRNAs to proteins of the Piwi subfamily ubiquitously existing in the reproductive system (Juliano et al., 2011). piRNAs, one of the richest types of small RNAs in the reproductive system of animals, were first discovered in the testes of Drosophila melanogaster about 20 years ago (Aravin et al., 2001). The discovery of piRNA was elected as one of the ten major scientific advances by Science in 2006, which has aroused the interest and attention of scientific researchers since.
piRNA precursors lack the specific sequence tags required to generate small RNAs. Theoretically, all gene transcripts can be converted to piRNA, but in reality, only certain locations in the genome are transcribed to generate piRNA, and such locations are called the piRNA cluster (Czech et al., 2018). Typically, piRNA clusters are transcribed to produce single-stranded piRNA precursors (primary piRNAs), which are then transported from the nucleus to the cytoplasm and processed there to bind to proteins of the Piwi family and enter the unique piRNA biogenesis pathway, i.e., the ping-pong amplification cycle to form mature piRNAs.
The evolution and reproductive activities of mammals are tightly regulated. The transposon is an important part of the genome of eukaryotic cells, and its activation will cause the arrest of germ cell development (Mani and Juliano, 2013). Therefore, normal gamete development depends on a rigid inhibition of transposon activity (Russell et al., 2017). piRNAs are reported to be abundantly expressed in germ cells, and they silence transposons and protect the genome from the impacts of transposons, thus maintaining the integrity and fertility of the germline (Di Giacomo et al., 2013). In the mammalian testes, piRNAs are expressed in a developmental stage-dependent manner and by coupling with proteins of the Piwi subfamily, they play a regulatory role at various stages of spermatogenesis in maintaining male fertility and genomic integrity (Girard et al., 2006; Di Giacomo et al., 2013; Kawase and Ichiyanagi, 2022). Spermatogenesis is a highly coordinated and tightly regulated process of cell development in which chromatin concentration results in the decoupling of transcription and translation until translation becomes necessary. It is well documented that piRNAs, in adult testis, regulate both mRNA translation and degradation during spermatogenesis to maintain normal germ cell development and morphological transformation of sperms (Dai et al., 2019). Interruption of the piRNA pathway and a loss of function of proteins of the Piwi family usually activates transposable elements (TEs), in turn causing spermatogenic arrest and male sterility (Dong et al., 2019; Choi et al., 2021). Also, piRNAs carry important functional roles in post-testicular sperm maturation and fertilization (Perillo et al., 2023). Additionally, piRNAs are widely expressed in somatic cells, and they are involved in regulating gene expression (Sun et al., 2022), controlling translation and mRNA stability (Sun et al., 2021), maintaining stem cell function (Rojas-Ríos and Simonelig, 2018), and regulating cell cycle progression (Mani and Juliano, 2013). As of today, piRNAs have been identified in the testes of model animals such as Drosophila (Siomi et al., 2010) and mice (Dai et al., 2019) and non-model animals such as pigs (Ma et al., 2022), horses (Li et al., 2019), yaks (La et al., 2022), and sheep (Di et al., 2022). These studies have shown that the expression pattern and regulatory role of piRNAs in the testis of mammals vary with species and the developmental stage.
Tibetan sheep (Ovis aries) is one of the famous domestic sheep breeds in China. It inhabits mainly in Qinghai-Tibet Plateau above 3,000 m above sea level. It not only provides important production and living materials for local farmers and herdsmen but also plays an important ecological role in Qinghai-Tibet Plateau (Niu et al., 2017). The characteristics and functions of piRNAs during testicular development of this species are unknown. In order to reveal the sequence characteristics, expression pattern, and biological function of piRNAs in the testis of Tibetan sheep at different developmental stages and to provide new insights into the mechanism of testicular development and spermatogenesis in sheep, piRNAs in the testis of Tibetan sheep at different developmental stages were identified by RNA sequencing technology in this study.
Materials and Methods
Ethics statement
All experiments were carried out in accordance with the guidelines from the Ethics Committee of the Laboratory Animal Center at Gansu Agricultural University (GSAU-Eth-ASF2022-008).
Animals and sample collection
Twelve healthy male Tibetan sheep used in this study were all provided by a breeding co-op (Xiahe County, Gansu Province) and they were at three developmental stages: before sexual maturation (3 months of age, n = 4), after sexual maturation (1 year of age, n = 4), and adulthood (3 years of age, n = 4). All testicular samples were collected in the autumn (October 29). They were sacrificed, and their right testicular tissues were harvested and rapidly separated into two parts: one was stored at −80°C for RNA extraction, and the remaining parts were fixed in 4% paraformaldehyde and embedded in paraffin for hematoxylin & eosin (H&E) staining using conventional methods.
Total RNA extraction
Total RNA was extracted from the tissues by using TRIzol Reagent, and the quality of RNAs was assessed by using agarose gel electrophoresis, NanoDrop 2000 spectrophotometer, and Agilent 2100 bioanalyzer (Agilent Technologies, USA). All samples had a RIN value above > 7.5 and were used for subsequent library construction and sequencing of small RNAs.
Construction of a small RNA library and RNA sequencing
Fragments of 18–45 nt in length were collected after agarose gel electrophoresis and then ligated with 5’ and 3’ linkers. The small RNAs containing both linkers were subject to reverse transcription and polymerase chain reaction (RT-PCR). Fragments of approximately 140–160 bp were recovered and purified by agarose gel electrophoresis and used for the construction of a library of small RNAs. Then RNA sequencing was performed on an Illumina HiSeq 2500 platform by Guangzhou Gene Denovo Biotechnology Co., Ltd.
Identification of piRNA
High-quality clean tags were obtained by first removing low-quality (Q ≤ 20) reads with unknown base N and linker sequences and inserts of < 18 nt in length from the original download data. The reads were aligned against the Genebank and Rfam databases by using the blast method, and small RNAs of rRNAs, tRNAs, snRNAs, scRNAs, and snoRNAs were eliminated. The reads were then aligned against the miRbase database by using the bowtie 2 software to eliminate known miRNAs. New miRNAs were identified using MiRdeep software and then eliminated. According to the characteristics of piRNAs, those of 24–33 nt in length and with the sequence characteristics of 1U/10 A were reserved as the candidate piRNA sequences.
piRNA cluster analysis
Since many piRNAs distribute in clusters and non-uniformly at specific genomic locations, a piRNA cluster analysis was performed using a window size of 5,000 bp for scanning the genome (the distance between two windows no more than 20K within the same piRNA cluster) and a criterion of not less than ten piRNAs per window. The expression abundance of piRNA clusters was normalized by calculation of TPM. The differentially expressed piRNA clusters were then identified with the criteria: |Fold change| > 2 and P value < 0.05.
Expression and functional enrichment analysis
The expression level of piRNAs in each sample was normalized by the TPM algorithm, and the piRNAs significantly differentially expressed between the groups were identified by |Fold change| > 2 and P value < 0.05. The target piRNA genes were predicted by using RNAhybrid, miRanda, and TargetScan software, and the intersection of the genes predicted by the software was deemed as the target piRNA genes. Gene Ontology (GO) and Kyoto Encyclopedia of Gene and Genomes (KEGG) databases were used for functional annotation and pathway enrichment analysis of the target genes.
qRT-PCR assay
The extracted RNAs (200 ng) were used as templates for reverse transcription using Mir-XTM miRNA First-Strand Synthesis Kit (Takara, Japan). Using U6 as an internal reference, TB GreenTM Fast qPCR Mix Kit (Takara, Japan), a two-step reaction procedure (95 °C 30s, 95 °C 5s, 60 °C 30s, 30 cycles) was used for qRT-PCR amplification on a Roche LightCycler 96 instrument (Switzerland). Each assay was repeated at least three times. The results were calculated using the 2–ΔΔCt method. The specific primers used were synthesized by Tsingke Biotechnology Co., Ltd. (China) and the sequence information is shown in Supplementary Table S1.
Statistical analysis
qRT-PCR data were analyzed by one-way ANOVA using SPSS 21.0 software and the results were expressed as mean ± standard deviation (mean ± SD). Group differences were analyzed by Duncan’s multiple-range test. *A P value < 0.05 is deemed statistically significant and ** a P value < 0.01 very significant, and ns (P > 0.05) means insignificant.
Results
Identification and characterization of piRNA sequences in testes of Tibetan sheep at different development stages
To begin with, H&E staining analysis indicated that, in the 3 months of age (3M) group, only spermatogonia and Sertoli cells were present in the seminiferous epithelium; in the 1 year of age (1Y) and 3 years of age (3Y) groups, the spermatogenic cells of all stages (spermatogonia, spermatocytes, and sperm) and Sertoli cells were distributed tightly and orderly in the seminiferous epithelium, exhibiting the increased number of spermatogenic cell layers and more abundant sperm in the 3Y group than those in the 1Y group (Supplementary Figure S1). Through small RNA sequencing, 54,224,307, 56,426,327, and 68,813,499 original small RNA reads were obtained in the 3M, 1Y, and 3Y groups, respectively, and 50,950,617, 43,594,011, and 49,591,408 high-quality clean reads were obtained after the screening. After screening out rRNAs, scRNAs, snoRNAs, snRNAs, tRNAs, and miRNAs, 137,846, 4,225,329, and 4,994,543 piRNA sequences were obtained in the 3M, 1Y, and 3Y groups, respectively. The length of the identified piRNAs ranged between 24 nt and 30 nt (Figure 1A). Sequence analysis showed strong uracil usage preferences (74.05%) at nucleotide 1 at the 5’ end, and increased uracil usage preferences at the 5’ end in testes after sexual maturation (1- and 3-year-old groups) compared to the 3-month-old group (Figure 1B and C), which indicates that the most piRNAs in Tibetan sheep testes are made by the phasing pathway (Ozata et al., 2019).
Figure 1.
Characteristics of the identified piRNA sequences. (A) Length distribution of piRNAs. (B) Frequency of nucleotide at each location in the piRNA sequences. (C) Frequency of uracil at the 5 ‘end of piRNAs in the testes at three developmental stages. 3M: 3 months of age; 1Y: 1 year of age; 3Y: 3 years of age.
Characteristics of the ping-pong structure and genomic distribution of piRNAs
The identified piRNA sequences have a distinct ping-pong structure (Figure 2A). Of these piRNAs, 28.96% were from exons in the gene region, 14.52% from repeat sequences in the intergene region, 10.99% from introns in the gene region, and 45.52% from other unannotated regions. The heat map showed that most piRNAs showed an expression in low abundance in the testis of Tibetan sheep, and the number of piRNAs with a high abundance of expression increased significantly with testicular development (Figure 2C).
Figure 2.
The ping-pong structure, distribution, and expression of piRNAs. (A) The Ping-pong structure. (B) Genomic distribution. (C) Heat map of piRNAs at different expression levels. The × axis represents the piRNA expression level (TPM value), and the number within the box is the number of piRNAs.
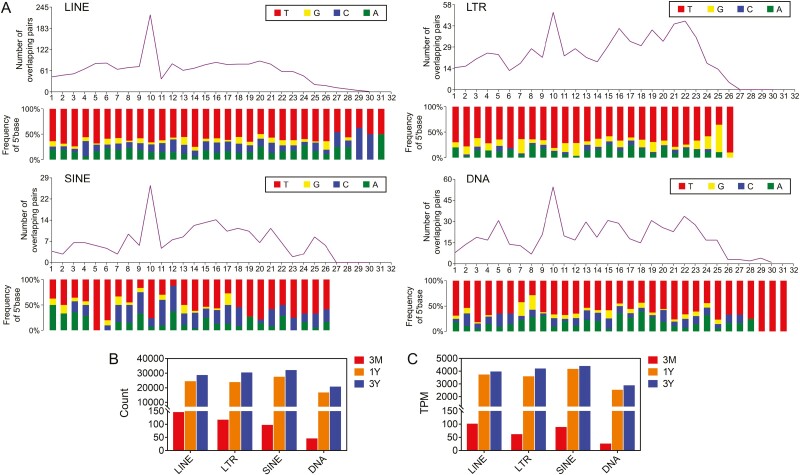
Characterization of TEs-derived piRNAs
According to Lu et al. (2010), the piRNAs derived from the genomic repeat region were aligned against the TE sequences using the Blastn software, and only the exact and complementary alignment results of the two were retained (three mismatches allowed). The results showed that 10.49% of piRNAs were aligned with TEs in the reference genome, and the total number of piRNAs aligned to its sense and antisense chains were almost the same. piRNAs showed a strong preference for long terminal repeat (LTR) and short interspersed elements (SINE) antisense strands and for long interspersed nuclear element (LINE) and DNA transposon sense strands. In the developing testicular tissues of the three age groups, the transposons with the greatest number of piRNA alignments were the retrotransposon LINE, followed by the retrotransposons LTR, SINE, and DNA transposon (Supplementary Table S2). Analysis of the piRNA sequences for each of the above TEs revealed an obvious ping-pong structure (Figure 3A), suggesting that piRNAs derived from each of the TEs may inhibit transposons via the ping-pong amplification cycle. In addition, when compared with the 3-month-old group, there were significant differences in the sequence populations and abundance of expression of piRNAs derived from each type of TEs in the testes of the 1- and 3-year groups, and the sequence populations and abundance of expression of piRNAs increased with testicular development (Figure 3B and C).
Figure 3.
Characteristics of piRNAs derived from different TE types. (A) The ping-pong structure of piRNAs derived from TEs. (B) Number of TE-derived piRNA sequences. (C) Expression abundance of TE-derived piRNAs.
Distribution characteristics of piRNA clusters on chromosomes
A total of 2,568 piRNA clusters were identified by cluster analysis and they distributed widely on each chromosome, but mainly on chromosomes 3 (260 clusters), 14 (199 clusters), 1 (193 clusters), 2 (185 clusters), 11 (182), 5 (158 clusters), 13 (127 clusters), and 24 (126 clusters) (Figure 4A). The differential expression analysis showed that a total of 529 piRNA clusters were differentially expressed in at least two stages across testis development (Figure 4B). Among these, compared with the 3-month-old group, 313 and 469 differential clusters were all upregulated in the 1-year-old group and 3-year-old group, respectively; compared with the 1-year-old group, the 3-year-old group had 42 differential clusters (23 upregulated; 19 downregulated) (Figure 4B and C); 25 differential piRNA clusters were co-expressed among all age groups, and 30 differential piRNA clusters were commonly shared by 3M vs. 1Y and 1Y vs. 3Y (Figure 4C).
Figure 4.
Chromosomal distribution and differential expression analysis of piRNA clusters. (A) Distribution maps of piRNA clusters on double-stranded chromosomal DNAs. (B) Heat map of differentially expressed piRNA clusters. (C) Venn diagram displaying the numbers of shared and unique piRNA clusters.
Expression and function analysis of piRNA target genes
In order to better understand the role of these candidate piRNAs in the testicular development of Tibetan sheep, their target genes were analyzed, and a total of 1,726 target genes were obtained. The mRNA sequencing data we collected previously suggested that most of these genes were down-regulated with testicular development (Figure 5A). The results of GO functional annotation of candidate piRNA target gene showed that in terms of biological processes, they are significantly enriched in metabolism, immunity, development, chromosome organization, and processes closely related to the biological function of testis such as assembly and morphogenesis of spermatozoa flagella. In terms of cell components, they are mainly involved in the events related to the connection/adhesion between cells or between cells and extracellular matrix, such as tight connection, apical connection complex, and lamellar adhesion complex. In terms of molecular function, they are associated with binding, motor activity, and kinase activity (Figure 5B). The KEGG pathway enrichment analysis showed that the target genes are predominantly enriched in pathways associated with development (e.g., cell cycle, MAPK, AMPK, and PI3K-Akt signaling pathways), reproduction (steroid biosynthesis, glycolysis/glucose production, estrogen, and Hippo signaling pathways), and maintenance of testicular immune privilege (e.g., ECM-receptor interaction, focal adhesion, and B-cell/T-cell receptor signaling pathways) (Figure 5C).
Figure 5.
Expression and functional enrichment analysis of piRNA target genes. (A) Heat map of target gene expression. (B) GO functional annotation analysis. (C) KEGG enrichment analysis.
Differential expression and function analysis of piRNAs
Differential expression analysis revealed a notable difference in candidate piRNA expression between groups: compared with the 3-month-old group, the 1-year-old group had 41,552 differential candidate piRNAs (41,374 upregulated; 178 downregulated); compared with the 3-month-old group, the 1-year-old group had 2,529 differential piRNAs (1,843 upregulated; 686 downregulated) (Figure 6A and B). Among these, 600 differential candidate piRNAs were co-expressed among all age groups, and 874 differential piRNAs were commonly shared in 3M vs. 1Y and 1Y vs. 3Y (Figure 6A). The cluster analysis revealed that differential candidate piRNAs could be categorized into four distinct expression patterns in developmental testes. The cluster 1 (9,021 candidate piRNAs) and cluster 4 (16,393 candidate piRNAs) exhibited an overall trend of first increasing and then decreasing, and the cluster 2 (8,487 candidate piRNAs) and cluster 3 (14,786 candidate piRNAs) exhibited an overall increasing trend with age (Figure 6C).
Figure 6.
Differential piRNA expression profiles and functional annotation. (A) Venn diagram depicting the shared and unique piRNAs. (B) piRNA expression profiles between 3M and 1Y groups (left panel), and 1Y and 3Y groups (right panel). (C) Cluster analysis showing similar piRNA expression patterns during development. (D) The significantly enriched GO functional annotation for putative piRNA targets.
Functional enrichment analysis showed that the target genes in 3M vs. 1Y were significantly enriched in GO terms related to the regulation of transcription (such as transcription from RNA polymerase III promoter and RNA polymerase III regulatory region DNA binding), post-translational modification (such as histone H4 acetylation, protein acylation, and acetyltransferase activity), cell cycle (such as G2 DNA damage checkpoint), and metabolism (such as cellular macromolecule metabolic process and protein metabolic process); target genes in 1Y vs. 3Y were significantly enriched in GO terms including regulation of the developmental process, regulation of mitochondrial fusion, multicellular organismal homeostasis, gene expression, and metabolism (such as cellular macromolecule metabolic process) (Figure 6D). Specifically, the two putative target genes (RNF10, RING finger protein 10; OMA1, overlapping with the m-AAA protease 1 homolog) for five candidate piRNAs were implicated in gene expression regulation, target MAF1 for three candidate piRNAs was implicated in transcription regulation, and seven targets (CAMKV, calmodulin kinase-like vesicle-associated gene; RNF10; OAS2, 2ʹ, 5ʹ-oligoadenylate synthase 2; LMTK3, lemur tyrosine kinase 3; SH3RF2, SH3 domain containing ring finger 2; PHF20, PHD finger protein 20; and TAOK3, TAO kinase 3) for 18 candidate piRNAs were implicated in multiple protein modification (Figure 7). For instance, RNF10 is implicated in the innate immune response (Cao et al., 2021); OMA1 is closely associated with semen trait (sperm morphology abnormality) (Zhao et al., 2020); MAF1, a central RNA polymerase III-associated transcription repressor, plays an essential role in response to adverse growth conditions, such as oxidative stress and DNA damage (Kim et al., 2021), and a recent study revealed that alterations in cellular physiology lead to adjustments in MAF1 transcription to balance metabolic energy, thereby helping to maintain appropriate levels of non-coding RNAs (Willis, 2018). These suggest that the candidate piRNAs may play important roles in transcription regulation, testicular cell development, and immune privilege via target genes, but the specific role and the mechanisms involved remain to be elucidated.
Figure 7.
Sankey diagram showing the representative piRNA-mRNA-GO biological process.
qRT-PCR validation
The results of the qRT-PCR validation of the randomly selected ten piRNAs showed that the expression of seven piRNAs was significantly upregulated in the 1-year-old and the 3-year-old groups compared with the 3-month-old group, while the expression of three piRNAs trended to be downregulated Figure 8 which was basically consistent with the sequencing results, indicating that the piRNA sequencing data were reliable.
Figure 8.
qRT-PCR validation of RNA-seq data. The data are presented as means with SD (mean ± SD). The P-value significance is indicated by **P, 0.01; *P, 0.05; ns, not significant.
Discussion
Previous studies have reported that piRNAs are enriched in the testes of mammals and the abundance of their expression depends on the developmental stage (Gebert et al., 2019; Li et al., 2019). In order to fully understand the expression of piRNAs and their potential biological function during testicular development of Tibetan sheep, the piRNA expression profile of Tibetan sheep testes was obtained by small RNA sequencing at three developmental stages, namely, before sexual maturation (3 months of age), after sexual maturation (1 year of age), and adulthood (3 years of age). It was found that the number of piRNAs increased significantly after sexual maturation compared with that before sexual maturation, suggesting that piRNAs may play an important role in the spermatogenesis of Tibetan sheep after sexual maturation. The length of most of the identified piRNAs varied between 24 nt and 30 nt, with a peak seen at 24 nt before sexual maturation. The distribution of piRNAs of various lengths tended to be even after sexual maturity and during adulthood, although the number of piRNAs of 24–26 nt and 29 nt in length was relatively great. Different piwi proteins tended to bind to piRNAs of different lengths, and it was found that piRNAs of 24 nt, 25 nt, 26 nt, 28 nt, and 30 nt in length preferentially bound to argonaute 3 (AGO3), aubergine (Aub), piwi‐like RNA‐mediated gene silencing 2 (Piwil2), piwi‐like RNA‐mediated gene silencing 4 (Piwil4), and piwi‐like RNA‐mediated gene silencing 1 (Piwil1) proteins, respectively (Théron et al., 2018; Gebert et al., 2019). Therefore, the difference in the number of piRNAs in the testis of different developmental stages may be associated with the type and abundance of piwi proteins expressed. In addition, the identified piRNAs showed a strong base usage preference for uracil at the 5’ end, which is consistent with the common characteristics of piRNAs and with the findings with the testes of Mongolian horses (Li et al., 2019) and chicken (Chang et al., 2018), suggesting that the majority of the identified piRNAs originate from the primary piRNA pathway. Previous studies have reported that testicular piRNAs tend to have adenine usage preferences at nucleotide 10 at their 5’ end during formation, which is related to the “ping-pong amplification cycle” of piRNA formation, as confirmed by the results of this study.
piRNAs derived from different genomic regions play different roles. Hence, we analyzed the distribution characteristics of the identified piRNAs in the genome. Our data suggest that in Tibetan sheep testes, 28.96% of piRNAs are derived from exon regions, 14.52% from repeat sequence-rich intergenic regions, 10.99% from intron regions, and 45.52% from unannotated regions, implying that piRNAs in Tibetan sheep testes play a broader role other than inhibition of transposon activity, such as regulating gene expression. To sum up, even in the same type of tissues, the genomic distribution of piRNAs varies among different species or breeds, which may be the prerequisite for them to play various biological functions, and this phenomenon also indicates the necessity of piRNA research in each sheep breed.
It is well known that many piRNAs in the reproductive system play an essential role in inhibiting the activity of TEs (Di Giacomo et al., 2013; Wang et al., 2023). To further explore the effect of piRNAs on TEs in Tibetan sheep testes, we aligned piRNAs derived from repeat sequence regions against TE sequences and identified four major TE types (LINE, LTR, SINE, and DNA transposons). Most of them were aligned to retrotransposons, which is consistent with previous studies in the yak (Gong et al., 2018), porcine (Ma et al., 2022), and human (Ha et al., 2014). Specifically, however, the piRNAs in human testes were mainly aligned to the LTRs of the retrotransposons, while those in the testes of Tibetan sheep, like pigs, yaks, and sheep, were mainly aligned to the LINE retrotransposon. Notably, piRNA sequences, regardless of the type of retrotransposon, have the typical ping-pong structure, implying that piRNAs in Tibetan sheep testicular tissue play an important role in silencing TEs to maintain the integrity of the genome. These results suggest that piRNAs may act on different targets in different species to inhibit the transposon activity and maintain testicular genomic stability. piRNAs have been reported to exist in clusters on chromosomes and to be non-uniformly distributed on chromosomes (Ma et al., 2022). Similarly, the piRNAs identified in this study were divided into 2,568 clusters, which are widely distributed on autosomes 1 to 26 and X chromosome. However, there are relatively more clusters on chromosomes 1, 2, 3, 5, 11, 13, 14, and 24 (all > 100), suggesting that piRNAs on these chromosomes play a more important role in maintaining genomic stability and biological function of the testis. Additionally, we identified a total of 529 differentially expressed piRNA clusters in developmental testis development, and the great majority of these clusters were upregulated with age, suggestive for their important roles in post-pubertal and adult Tibetan sheep testes.
Previous studies have shown that the expression and function of piRNAs vary with the developmental stage (Kawase and Ichiyanagi, 2022). To identify differences in the abundance of expression and the biological function of candidate piRNAs in the testes of developing Tibetan sheep, we identified differentially expressed candidate piRNAs in the testes at different stages of development. Totally, 41,552 and 2,529 candidate piRNAs were differentially expressed in the testes of 3M vs. 1Y and 1Y vs. 3Y groups, respectively, and the vast majority of candidate piRNAs presented an increased abundance in the latter, indicative of indispensable functional roles in spermatogenesis. To further understand the potential role of these candidate piRNAs, we performed a functional enrichment analysis of their target genes. For 3M vs. 1Y, the targets were principally involved in regulating the transcription such as transcription from RNA polymerase III promoter, and RNA polymerase III regulatory region DNA binding; post-translational modification such as histone H4 acetylation, protein acylation, and acetyltransferase activity; G2 DNA damage checkpoint; metabolism such as cellular macromolecule metabolic process, and protein metabolic process. For 1Y vs. 3Y, target genes were implicated in regulation of developmental process, multicellular organismal homeostasis, gene expression. RNA polymerase III is the most complex RNA polymerase that is known to be involved in RNA transcription in eukaryotic cells (Turowski and Tollervey, 2016). Spermatogenesis is a prolonged and highly ordered cell differentiation process that is regulated by complex factors including post-translational protein modifications (Suresh et al., 2015; O’Flaherty and Matsushita-Fournier, 2017). Protein acetylation is a major post-translational modification in the epigenetic regulation of various biological processes including the reproductive process (Pang and Rennert, 2013; Qian et al., 2013). It has been well demonstrated that acetylation modifications continue throughout spermatogenesis contributing to germ cell development through inducing the well-timed release of stored mRNA transcripts for translation and modulating the cellular localization of target proteins (Pang and Rennert, 2013). For instance, histone H4 acetylation functions in chromatin remodeling during spermiogenesis to produce fertile spermatozoa (Ketchum et al., 2018). DNA damage can result in cell cycle arrest or delay before the S phase, during replication, and before mitosis, whereas damage checkpoint is an important mechanism in cell cycle regulation for precise cell division and cell survival as well as genome stability (Calonge and O’Connell, 2008). The above findings suggest that these differential candidate piRNAs may be widely participated in the functional regulation of germ cell development so as to ensure the normal progression of spermatogenesis in Tibetan sheep, though the precise mechanism still remained to be further evaluated. Although the complementarity between piRNA and target gene is stable, it is important to note that 3ʹ terminal 2’-O-methylation is required for this stability (Capra et al., 2017). Therefore, whether the candidate piRNAs can bind to PIWI proteins to cleave target genes, requires further validation.
Conclusion
In conclusion, we obtained the piRNA expression profile of the testis of Tibetan sheep at different developmental stages and revealed the characteristics of these piRNAs, including their length distribution, genomic distribution, ping-pong structure, and chromosome distribution. Most piRNAs had a low expression abundance in the developing testes of Tibetan sheep. Many differentially expressed candidate piRNAs were significantly upregulated in the testes after sexual maturation, and functional annotations based on target genes suggest that these differentially expressed candidate piRNAs regulate Tibetan sheep spermatogenesis mainly by participating in the regulation of gene expression, transcription, protein modification, and cell development. These findings contribute to a better understanding of the role of piRNAs in the maintenance of male fertility and provide new insights into the regulatory mechanism of spermatogenesis in sheep.
Supplementary Data
Supplementary data are available at Journal of Animal Science online.
Acknowledgments
We are grateful for assisting in RNA sequencing provided by Genedenovo Biotechnology Co., Ltd (Guangzhou, China). This work was financially supported by the Education Science and Technology Innovation Project of Gansu Province (GSSYLXM-02), Gansu Provincial Department of Education: Young Doctor Fund Project (2022QB-074), “Innovation Star” project for outstanding graduate students of the Education Department of Gansu Province (2023CXZX-631 and 2022CXZX-628), National Natural Science Foundation of China (32202675 and 32260833), Youth Science and Technology Foundation of Gansu Province (No. 22JR5RA886), Scientific Research Start-up Funds for Openly-recruited Doctors (GAU-KYQD-2021-06), and Discipline Team Project of Gansu Agricultural University (GAU-XKTD-2022-20).
Glossary
Abbreviations:
- 1Y
1 year of age
- 3M
3 months of age
- 3Y
3 years of age
- AGO3
argonaute 3
- Aub
aubergine
- GO
Gene Ontology
- H&E
hematoxylin and eosin
- KEGG
Kyoto Encyclopedia of Gene and Genomes
- LINE
long interspersed nuclear element
- LTR
long terminal repeat
- miRNAs
microRNAs
- OMA1
overlapping with the m-AAA protease 1 homolog
- piRNAs
PIWI-interacting RNAs
- Piwil2
piwi-like RNA-mediated gene silencing 2
- Piwil1
piwi-like RNA-mediated gene silencing 1
- Piwil4
piwi-like RNA-mediated gene silencing 4
- RNF10
RING finger protein 10
- RT-PCR
reverse transcription and polymerase chain reaction
- SINE
short interspersed element
- siRNAs
small interfering RNAs
- TEs
transposable elements
Contributor Information
Taotao Li, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou 730070, China; Gansu Key Laboratory of Animal Generational Physiology and Reproductive Regulation, Lanzhou 730070, China.
Huihui Wang, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou 730070, China; Gansu Key Laboratory of Animal Generational Physiology and Reproductive Regulation, Lanzhou 730070, China.
Keyan Ma, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou 730070, China.
Yi Wu, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou 730070, China.
Xingcai Qi, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou 730070, China.
Zilong Liu, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou 730070, China.
Qiao Li, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou 730070, China.
Yong Zhang, Gansu Key Laboratory of Animal Generational Physiology and Reproductive Regulation, Lanzhou 730070, China.
Youji Ma, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou 730070, China; Gansu Key Laboratory of Animal Generational Physiology and Reproductive Regulation, Lanzhou 730070, China.
Data Availability
The raw RNA-seq data reported in this article have been deposited in the NCBI SRA database (SRA ID: SRP253288) that are publicly accessible at https://www.ncbi.nlm.nih.gov/sra.
Conflict of interest statement. All authors declare no competing or conflicts of interests.
Literature Cited
- Aravin, A., Naumova N., Tulin A., Vagin V., Rozovsky Y., and Gvozdev V.. . 2001. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 11:1017–1027. doi: 10.1016/s0960-9822(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Calonge, T., and O’Connell M.. . 2008. Turning off the G2 DNA damage checkpoint. DNA Repair. 7:136–140. doi: 10.1016/j.dnarep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., Liu L., Zhang Y., and Yang Y.. . 2021. Reduced RING finger protein 10 expression in macrophages is associated with aging-related inflammation. FEBS Open Bio. 11:386–394. doi: 10.1002/2211-5463.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra, E., Turri F., Lazzari B., Cremonesi P., Gliozzi T., Fojadelli I., Stella A., and Pizzi F.. . 2017. Small RNA sequencing of cryopreserved semen from single bull revealed altered miRNAs and piRNAs expression between high- and low-motile sperm populations. BMC Genom. 18:14. doi: 10.1186/s12864-016-3394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, K., Tseng Y., Chen Y., Yu C., Liao H., Chen Y., Tu Y., Wu S., Liu I., Pinskaya M., . et al. 2018. Stage-dependent piRNAs in chicken implicated roles in modulating male germ cell development. BMC Genoms. 19:425. doi: 10.1186/s12864-018-4820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, H., Wang Z., and Dean J.. . 2021. Sperm acrosome overgrowth and infertility in mice lacking chromosome 18 pachytene piRNA. PLoS Genet. 17:e1009485. doi: 10.1371/journal.pgen.1009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech, B., Munafò M., Ciabrelli F., Eastwood E., Fabry M., Kneuss E., and Hannon G.. . 2018. piRNA-guided genome defense: from biogenesis to silencing. Annu. Rev. Genet. 52:131–157. doi: 10.1146/annurev-genet-120417-031441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, P., Wang X., Gou L., Li Z., Wen Z., Chen Z., Hua M., Zhong A., Wang L., Su H., . et al. 2019. A translation-activating function of MIWI/piRNA during mouse spermiogenesis. Cell. 179:1566–1581.e16. doi: 10.1016/j.cell.2019.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, R., Zhang R., Mwacharo J., Wang X., He X., Liu Y., Zhang J., Gong Y., Zhang X., and Chu M.. . 2022. Characteristics of piRNAs and their comparative profiling in testes of sheep with different fertility. Front. Genet. 13:1078049. doi: 10.3389/fgene.2022.1078049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giacomo, M., Comazzetto S., Saini H., De Fazio S., Carrieri C., Morgan M., Vasiliauskaite L., Benes V., Enright A., and O’Carroll D.. . 2013. Multiple epigenetic mechanisms and the piRNA pathway enforce LINE1 silencing during adult spermatogenesis. Mol. Cell. 50:601–608. doi: 10.1016/j.molcel.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Dong, J., Wang X., Cao C., Wen Y., Sakashita A., Chen S., Zhang J., Zhang Y., Zhou L., Luo M., . et al. 2019. UHRF1 suppresses retrotransposons and cooperates with PRMT5 and PIWI proteins in male germ cells. Nat. Commun. 10:4705. doi: 10.1038/s41467-019-12455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert, D., Zischler H., and Rosenkranz D.. . 2019. Primate piRNA cluster evolution suggests limited relevance of pseudogenes in piRNA-mediated gene regulation. Genome Biol. Evol. 11: 1088–1104. doi: 10.1093/gbe/evz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard, A., Sachidanandam R., Hannon G., and Carmell M.. . 2006. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 442:199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- Gong, J., Zhang Q., Wang Q., Ma Y., Du J., Zhang Y., and Zhao X.. . 2018. Identification and verification of potential piRNAs from domesticated yak testis. Reproduction. 155:117–127. doi: 10.1530/REP-17-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, H., Song J., Wang S., Kapusta A., Feschotte C., Chen K., and Xing J.. . 2014. A comprehensive analysis of piRNAs from adult human testis and their relationship with genes and mobile elements. BMC Genom. 15:545. doi: 10.1186/1471-2164-15-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano, C., Wang J., and Lin H.. . 2011. Uniting germline and stem cells: the function of Piwi proteins and the piRNA pathway in diverse organisms. Annu. Rev. Genet. 45:447–469. doi: 10.1146/annurev-genet-110410-132541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase, M., and Ichiyanagi K.. . 2022. The expression dynamics of piRNAs derived from male germline piRNA clusters and retrotransposons. Front. Cell Dev. Biol. 10:868746. doi: 10.3389/fcell.2022.868746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum, C., Larsen C., McNeil A., Meyer-Ficca M., and Meyer R.. . 2018. Early histone H4 acetylation during chromatin remodeling in equine spermatogenesis. Biol. Reprod. 98:115–129. doi: 10.1093/biolre/iox159. [DOI] [PubMed] [Google Scholar]
- Kim, J., Cho J., Kim J., Kim D., Nam B., Kim B., Kim W., Kim Y., Cheong J., and Kong H.. . 2021. Molecular characterization of Paralichthys olivaceus MAF1 and its potential role as an anti-viral hemorrhagic septicaemia virus factor in hirame natural embryo cells. Int. J. Mol. Sci. 22:1353. doi: 10.3390/ijms22031353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La, Y., Ma X., Bao P., Chu M., Yan P., Guo X., and Liang C.. . 2022. Identification and characterization of piwi-interacting RNAs for early testicular development in yak. Int. J. Mol. Sci. 23:12320. doi: 10.3390/ijms232012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B., He X., Zhao Y., Bai D., Bou G., Zhang X., Su S., Dao L., Liu R., Wang Y., . et al. 2019. Identification of piRNAs and piRNA clusters in the testes of the Mongolian horse. Sci. Rep. 9:5022. doi: 10.1038/s41598-019-41475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Carmell M., Rivas F., Marsden C., Thomson J., Song J., Hammond S., Joshua-Tor L., and Hannon G.. . 2004. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Lu, J., and Clark A.. . 2010. Population dynamics of PIWI-interacting RNAs (piRNAs) and their targets in Drosophila. Genome Res. 20:212–227. doi: 10.1101/gr.095406.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X., Niu X., Huang S., Li S., Ran X., Wang J., and Dai X.. . 2022. The piRNAs present in the developing testes of Chinese indigenous Xiang pigs. Theriogenology. 189:92–106. doi: 10.1016/j.theriogenology.2022.05.028. [DOI] [PubMed] [Google Scholar]
- Mani, S., and Juliano C.. . 2013. Untangling the web: the diverse functions of the PIWI/piRNA pathway. Mol. Reprod. Dev. 80:632–664. doi: 10.1002/mrd.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister, G. 2013. Argonaute proteins: functional insights and emerging roles. Nat. Rev. Genet. 14:447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- Niu, L., Chen X., Xiao P., Zhao Q., Zhou J., Hu J., Sun H., Guo J., Li L., Wang L., . et al. 2017. Detecting signatures of selection within the Tibetan sheep mitochondrial genome. Mitochondrial DNA Part A. 28:801–809. doi: 10.1080/24701394.2016.1192614. [DOI] [PubMed] [Google Scholar]
- O’Flaherty, C., and Matsushita-Fournier D.. . 2017. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 97:577–585. doi: 10.1093/biolre/iox104. [DOI] [PubMed] [Google Scholar]
- Ozata, D., Gainetdinov I., Zoch A., O’Carroll D., and Zamore P.. . 2019. PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet. 20:89–108. doi: 10.1038/s41576-018-0073-3. [DOI] [PubMed] [Google Scholar]
- Pang, A., and Rennert O.. . 2013. Protein acetylation and spermatogenesis. Reprod. Syst. Sex. Disord. Suppl 1: 5. doi: 10.4172/2161-038x.s1-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillo, G., Shibata K., and Wu P.. . 2023. piRNAs in sperm function and embryo viability. Reproduction. 165:R91–R102. doi: 10.1530/REP-22-0312. [DOI] [PubMed] [Google Scholar]
- Qian, M., Pang Y., Liu C., Haratake K., Du B., Ji D., Wang G., Zhu Q., Song W., Yu Y., . et al. 2013. Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell. 153:1012–1024. doi: 10.1016/j.cell.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Ríos, P., and Simonelig M.. . 2018. piRNAs and PIWI proteins: regulators of gene expression in development and stem cells. Development. 145:dev161786. doi: 10.1242/dev.161786. [DOI] [PubMed] [Google Scholar]
- Russell, S., Stalker L., and LaMarre J.. . 2017. PIWIs, piRNAs and retrotransposons: complex battles during reprogramming in gametes and early embryos. Reprod. Domest. Anim. Suppl 4: 28–38. doi: 10.1111/rda.13053. [DOI] [PubMed] [Google Scholar]
- Siomi, M., Miyoshi T., and Siomi H.. . 2010. piRNA-mediated silencing in Drosophila germlines. Semin. Cell Dev. Biol. 21:754–759. doi: 10.1016/j.semcdb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Sun, Y., Lee B., and Li X.. . 2022. The birth of piRNAs: how mammalian piRNAs are produced, originated, and evolved. Mamm. Genome. 33:293–311. doi: 10.1007/s00335-021-09927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y., Wang R., Du K., Zhu J., Zheng J., Xie L., Pereira A., Zhang C., Ricci E., and Li X.. . 2021. Coupled protein synthesis and ribosome-guided piRNA processing on mRNAs. Nat. Commun. 12:5970. doi: 10.1038/s41467-021-26233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh, B., Lee J., Hong S., Kim K., and Ramakrishna S.. . 2015. The role of deubiquitinating enzymes in spermatogenesis. Cell. Mol. Life Sci. 72:4711–4720. doi: 10.1007/s00018-015-2030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théron, E., Maupetit-Mehouas S., Pouchin P., Baudet L., Brasset E., and Vaury C.. . 2018. The interplay between the Argonaute proteins Piwi and Aub within Drosophila germarium is critical for oogenesis, piRNA biogenesis and TE silencing. Nucleic Acids Res. 46:10052–10065. doi: 10.1093/nar/gky695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson, T., and Lin H.. . 2009. The biogenesis and function of PIWI proteins and piRNAs: progress and prospect. Annu. Rev. Cell Dev. Biol. 25:355–376. doi: 10.1146/annurev.cellbio.24.110707.175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski, T., and Tollervey D.. . 2016. Transcription by RNA polymerase III: insights into mechanism and regulation. Biochem. Soc. Trans. 44:1367–1375. doi: 10.1042/bst20160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Ramat A., Simonelig M., and Liu M.. . 2023. Emerging roles and functional mechanisms of PIWI-interacting RNAs. Nat. Rev. Mol. Cell Biol. 24:123–141. doi: 10.1038/s41580-022-00528-0. [DOI] [PubMed] [Google Scholar]
- Willis, I. 2018. Maf1 phenotypes and cell physiology. Biochim. Biophys. Acta, Gene Regul. Mech. 1861:330–337. doi: 10.1016/j.bbagrm.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q., Li R., Lyu Q., Hou L., Liu Z., Sun Q., Liu M., Shi H., Xu B., Yin M., . et al. 2019. Single-cell CAS-seq reveals a class of short PIWI-interacting RNAs in human oocytes. Nat. Commun. 10:3389. doi: 10.1038/s41467-019-11312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y., Gao N., Li X., El-Ashram S., Wang Z., Zhu L., Jiang W., Peng X., Zhang C., Chen Y., . et al. 2020. Identifying candidate genes associated with sperm morphology abnormalities using weighted single-step GWAS in a Duroc boar population. Theriogenology. 141:9–15. doi: 10.1016/j.theriogenology.2019.08.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw RNA-seq data reported in this article have been deposited in the NCBI SRA database (SRA ID: SRP253288) that are publicly accessible at https://www.ncbi.nlm.nih.gov/sra.
Conflict of interest statement. All authors declare no competing or conflicts of interests.