Figure 8.
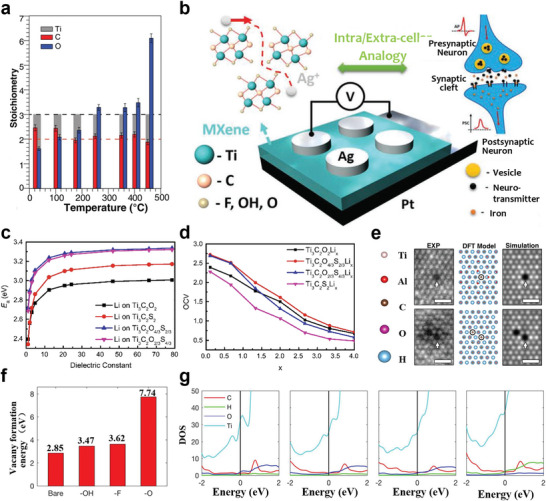
a) C/Ti/O stoichiometry quantitated by EELS from room temperature to 450 °C. Reproduced with permission.[ 141b ] Copyright 2020, Wiley‐VCH. b) The fabricated device in Ag/Ti3C2T x NS/Pt structure. Ag/Ti3C2T x NS/Pt device structure. Ag migration via MXene nanosheets are similar to ion migration in biological synapses. Reproduced with permission.[ 167 ] Copyright 2020, Wiley‐VCH. c) The adsorption energies (E a) as a function of dielectric constant in Ti3C2T2 (T = O or S) and Ti3C2O y S2− y (y = 4/3 and 2/3). d) The open‐circuit voltage (OCV) as a function of Ti3C2O2Li x , Ti3C2O4/3S2/3Li x , Ti3C2O2/3S4/3Li x , and Ti3C2S2Li x . Reproduced with permission.[ 168a ] Copyright 2020, Elsevier. e) Atomic‐scale high angle annular dark field‐Scanning transmission electron microscopy (HAADF‐STEM) images of defects in single‐layer Ti3C2T x . Comparison between experimental HAADF‐STEM image, defect crystal structure determined from DFT, and simulated HAADF‐STEM image of VTi and two adjacent VTi within the same sublayer. f) VTi formation energy on bare Ti3C2 and terminated single‐layer Ti3C2T x . g) Calculated DOS of —OH‐terminated Ti3C2 monolayer with pristine, VTi2, VTi4, and VTi6 cluster surface (from left to right). Reproduced with permission.[ 173 ] Copyright 2016, Royal Scoiety of Chemistry.
