Fig. 4.
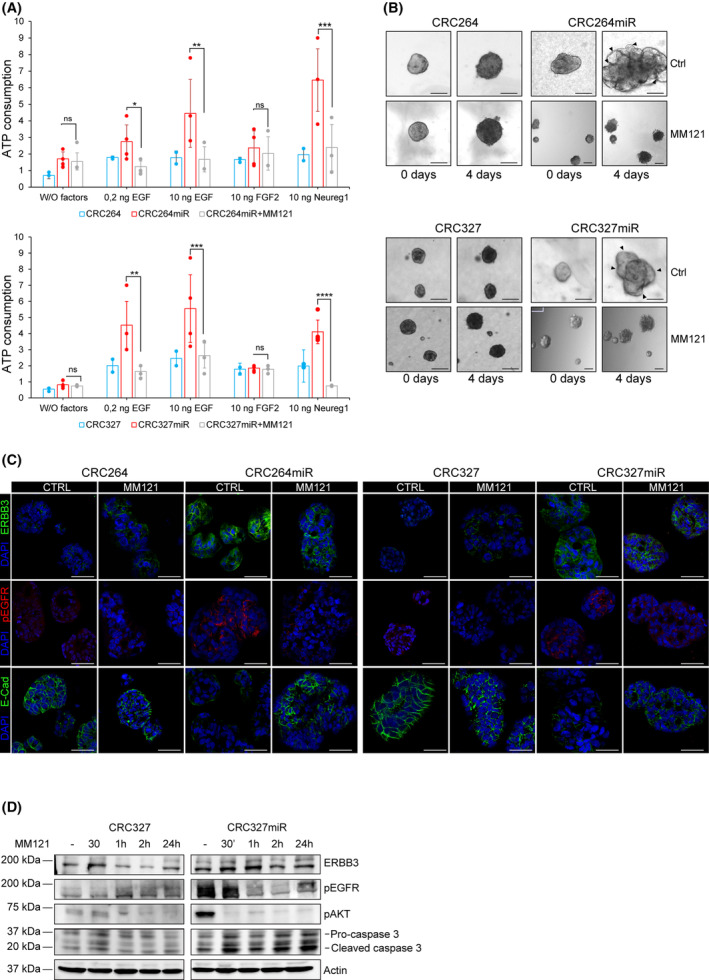
Selective ERBB3 inhibition dampens miRNA‐483‐3p‐induced invasive growth. (A) Viability of m‐colospheres (upper panel: CRC264 and CRC264miR; lower panel: CRC327 and CRC327 miR) kept for 4 days in basal medium (without growth factors: W/O factors), or in basal medium with EGF (0.2 or 10 ng·mL−1) or FGF2 (10 ng·mL−1) or neuregulin 1 (10 ng·mL−1). M‐colospheres overexpressing miRNA‐483‐3p were treated with MM121 (50 ng·mL−1). Bars represent ATP consumption, fold change at day 4 vs. day 0 ± SEM (n > 3; *, P = 0.05; **, P < 0.001; ***, P < 0.005; ****, P < 0.0001; one‐way ANOVA). (B) 3D‐spheroid invasion assay. M‐colospheres (upper panel: CRC264 and CRC264miR; lower panel: CRC327 and CRC327miR) were embedded in a matrigel‐collagen type I matrix in the absence of growth factors, with or without MM121 (50 ng·mL−1). Their growth was monitored by time‐lapse microscopy at the indicated time points (n = 3). Arrowheads: cell protrusion or dissociation from the spheroid surface. Scale bar, 50 μm. Quantitative morphometric analysis of invaded areas is shown in Fig. S4B. (C) Representative immunofluorescent stainings of ERBB3, phospho‐EGFR (pEGFR) and E‐cadherin in CRC264, CRC264miR, CRC327, CRC327miR m‐colospheres grown in absence or presence of MM121 (50 ng·mL−1) for 24 h. Nuclei were counterstained with DAPI. Scale bar, 50 μm (n = 3). (D) Representative western blot of ERBB3, pEGFR, pAKT, caspase 3 in CRC327 and CRC327miR after MM121 treatment (50 ng·mL−1). M‐colospheres were collected at the indicated time points (n = 3).
