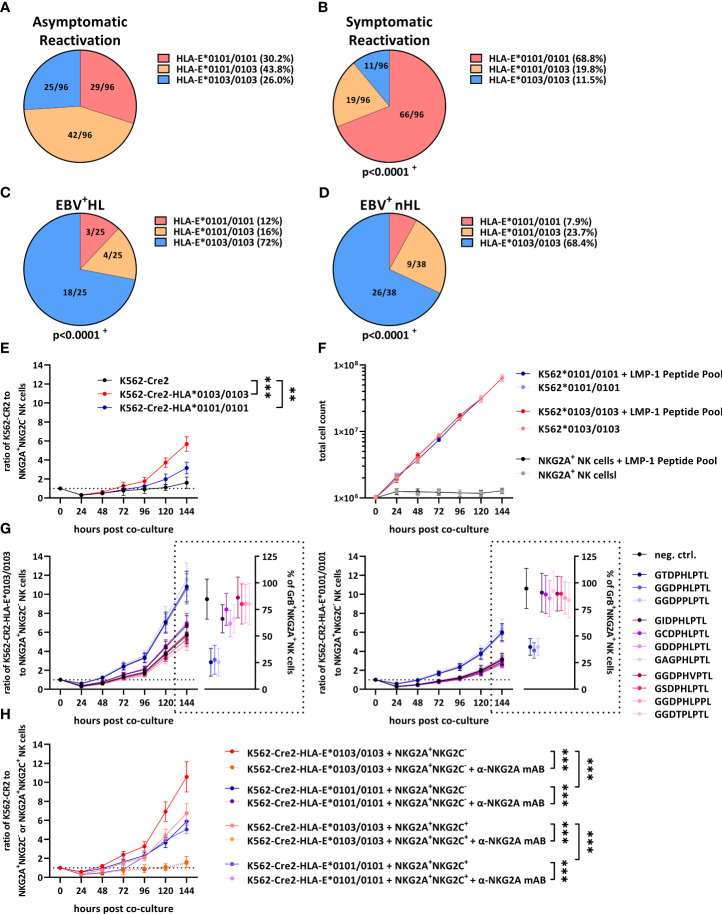
Figure 2.
HLA-E variants are associated with the development of EBV+ lymphomas and inhibit NKG2A+ NK cells. (A–D) Distribution of HLA-E variants in patients with (A) asymptomatic reactivations (N=96), (B) symptomatic reactivations (N=96), (C) EBV+HL (N=25), (D) EBV+nHL (N=38). Fractions represent the relative frequency of HLA-E*0101/0101, HLA-E*0101/0103 and HLA-E*0103/0103. + The frequency of the HLA-E variants was compared to the asymptomatic cohort by the Chi2 Test. (E–G) Cell proliferation assays. (E) Enriched NKG2A+NKG2C- NK cells were co-cultured with EBV-infected K562-CR2, K562-CR2-HLA-E*0103/0103 or K562-CR2-HLA-E*0101/0101 cells and were subsequently analyzed by flow-cytometry. (F) EBV-infected K562-CR2-HLA-E*0103/0103 or K562-CR2-HLA-E*0101/0101 cells or enriched NKG2A+NKG2C- NK cells were co-cultured an LMP-1 peptide pool containing 300 µM of each of the LMP-1 peptide-derived GGDPHLPTL, GSDPHLPTL, GGDPHLPPL, GGDPPLPTL, GCDPHLPTL, GIDPHLPTL, GAGPHLPTL, GGDTPLPTL, GDDPHLPTL, GGDPHVPTL and GTDPHLPTL variants. Viable EBV-infected K562-CR2-HLA-E*0103/0103 or K562-CR2-HLA-E*0101/0101 cells or enriched NKG2A+NKG2C- NK cells was subsequently analysed by flow-cytometry. (G) Enriched NKG2A+NKG2C- NK cells were co-cultured with EBV-infected K562-CR2, K562-CR2-HLA-E*0103/0103 or K562-CR2-HLA-E*0101/0101 cells and 300µM of the positive control (VMAPRTLFL) or the LMP-1 derived GGDPHLPTL, GSDPHLPTL, GGDPHLPPL, GGDPPLPTL, GCDPHLPTL, GIDPHLPTL, GAGPHLPTL, GGDTPLPTL, GDDPHLPTL, GGDPHVPTL and GTDPHLPTL and were subsequently analysed by flow-cytometry. (H) Enriched NKG2A+NKG2C- or NKG2A+NKG2C+ NK cells were co-cultured with EBV-infected K562-CR2, K562-CR2-HLA-E*0103/0103 or K562-CR2-HLA-E*0101/0101 cells and 300µM of the GGDPHLPTL variant and were subsequently analysed by flow-cytometry. For some experiments the α-NKG2A mAB 10µg/mL Monalizumab was added. (E–G) Plots represent the mean ( ± SD) of 12 independent replicates. RM one-way ANOVA (with the Geisser-Greenhouse correction) was used to compare the respective groups. p < 0.05 was considered significant. *p < 0.05; **p < 0.01; ***p < 0.001. EBV+HL, EBV+ Hodgkin lymphomas, EBV+nHL, EBV+ non-Hodgkin lymphomas, GrB, granzyme B, mAB, monoclonal antibody. Pos. Ctrl., positive control.