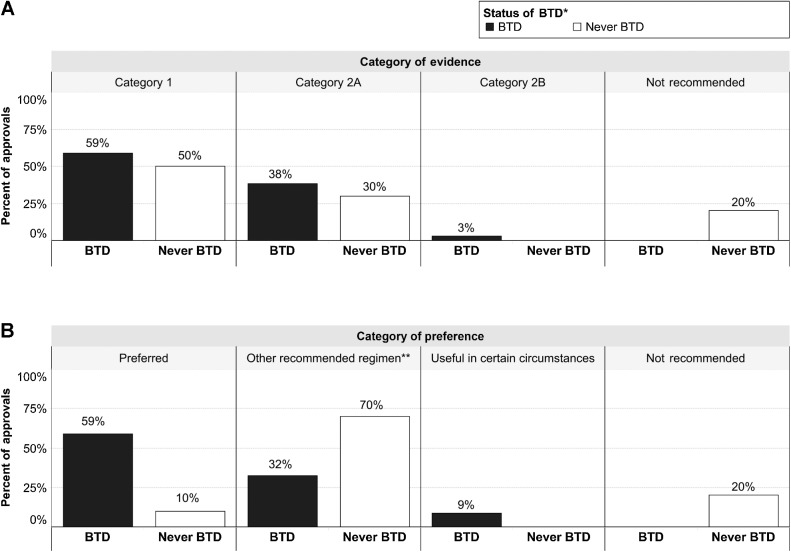
Figure 2.
Characteristics of NCCN recommendations for NSCLC approvals from 2013 to 2021. A, Percentage of BTD approvals and percentage of Never BTD approvals by category of evidence. B, Percentage of BTD approvals and percent of Never BTD approvals by category of preference. *“BTD”, approvals for a drug or a combination of drug(s) including drugs that have ever received BTD for any indication; “Never BTD”, approvals for drugs that have never received BTD for any indication. **Other recommended regimens are uses that are more toxic, less affordable, less efficacious, and/or are based on less mature data (8).