Abstract
Description
Enterococcus cecorum rarely serves as a human pathogen, and only 6 cases of this microbe inoculating humans have been documented. We present an elderly female with a marked vascular history presenting with epigastric pain and diarrhea, followed by shaking chills. Laboratory findings revealed leukocytosis, with imaging showing diffuse colonic thickening. She had a bout of bloody diarrhea, raising the likelihood of ischemic colitis with her vascular history. To our surprise, both sets of blood cultures drawn on admission grew Enterococcus cecorum. This case is unique as it is the first documented case of E. cecorum human infection in the United States.
Keywords: Enterococcus, Enterococcus cecorum, infectious disease, gastroenterology, bacterial infections, microbiology, infectious colitis, gram-positive bacterial infections, gram-positive bacterial infections/diagnosis, bacteremia
Introduction
Enterococcus cecorum (E. cecorum) is a gram-positive cocci bacterium that is found in the intestinal tracts of many domestic animals and poultry.1 Unlike Enterococcus faecalis that is found in more than 90 percent of human intestinal tracts, E. cecorum rarely serves as a human pathogen, with documented cases scarce.1 E. cecorum is not a known inhabitant of a human’s gastrointestinal tract, urinary tract or skin.2 Upon review of medical literature, only 6 cases of E. cecorum inoculating humans with clinically significant disease were found. These prior reported cases include patients with peritonitis, endocarditis, pulmonary abscess and septicemia.2–6 The portal of entry for this organism has not been studied in detail, but limited data show the possibility of a gastrointestinal source.7 We present an elderly female with a striking vascular history presenting with epigastric pain and diarrheal episode followed by fever and shaking chills found to have bacteremia caused by E. cecorum.
Case Presentation
We present a case of an 80-year-old female with a past medical history of infrarenal abdominal aortic aneurysm status post repair, thoracic aortic aneurysm, lung cancer status post lobectomy, peptic ulcer disease, chronic obstructive pulmonary disease, coronary artery disease status post cardiac stenting and ischemic colitis. The patient presented with intermittent, non-reproducible epigastric pain for the past 24 hours. The epigastric pain intermittently radiated between her shoulders and was aggravated by anxiety and stress. This pain was anxiety provoking to the patient as she had similar complaints in the past and ended up having life-threatening situations. In 2016, she reported a similar presentation and was found to have a superior mesenteric artery (SMA) occlusion and underwent stenting. In 2017, she reported having an episode of severe gastric ulcer bleeding that required a massive transfusion protocol and intervention via esophagogastroduodenoscopy. Furthermore, she reported a known 6.9 cm thoracic and abdominal aortic aneurysm that was deemed inoperable.
During this visit, the epigastric pain was associated with lightheadedness, shortness of breath and constipation. She became particularly concerned during the evening of her presentation when she had an episode of non-bloody diarrhea described as ‘explosive’ that was followed by the development of a subjective fever and shaking chills. This episode prompted her to present to the emergency department. Her initial vital signs showed a temperature of 98.4° F, a heart rate of 58 beats per minute, blood pressure of 98/58 mmHg and oxygen saturation of 95% on 2 L nasal cannula. Abnormal laboratory findings are shown in Table 1. Otherwise, her complete blood count, comprehensive metabolic panel and urinalysis were unremarkable.
Table 1.
Emergency Department Abnormal Laboratory Findings.
| Test | Patient’s value | Reference range |
|---|---|---|
| Serum leukocyte count | 14.2 × 109/L | 4.8–10.8 × 109/L |
| Neutrophil % of leukocyte count | 87.1% | 45–75% |
| Creatinine | 1.76 mg/dl (acute) | 0.6–1.2 mg/dl |
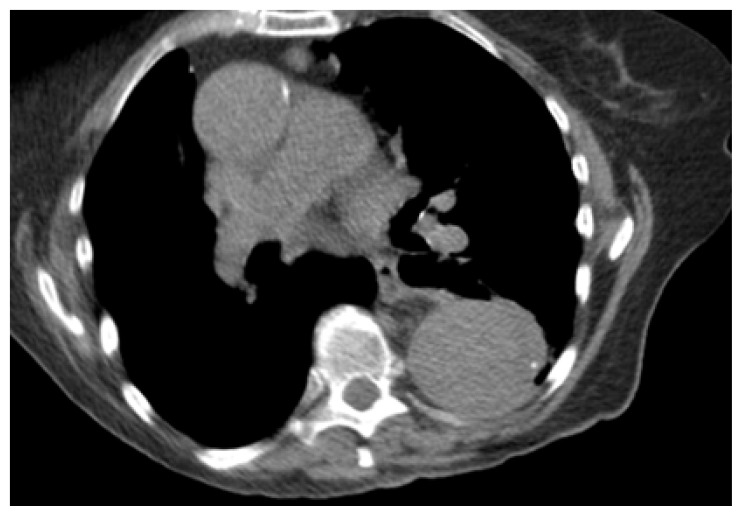
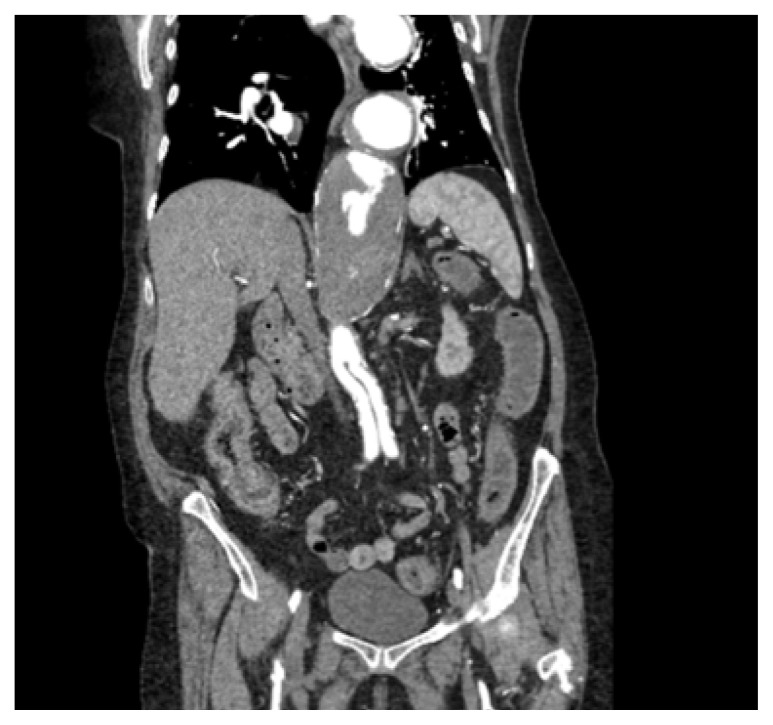
Due to the patient’s pertinent history of multiple vascular comorbidities, a computed tomography angiogram of her chest (CTA) and abdomen/pelvis was completed. (Figures 1 and 2) The massively enlarged thoracic aorta extended down into the left renal artery where an aortic graft was placed. There was no evidence of hematoma, gas, inflammation or any significant change from the same imaging study 1 year prior.
Figure 1.
Emergency Department CTA, Chest.
Figure 2.
Emergency Department CTA, Abdomen/Pelvis.
CT imaging of the abdomen/pelvis revealed diffuse mild thickening of the colonic wall, considerably sparing the cecum with liquid stool throughout. With the possibility of an infectious etiology as the source of the patient’s presentation, 2 sets of blood and stool cultures were collected, and empiric coverage with piperacillin/tazobactam was started. A urinalysis was negative for leukocyte esterase, nitrites or white blood cells. Gastroenterology was consulted, and the patient was admitted to the progressive care unit.
In less than 24 hours, both blood cultures began to grow gram-positive cocci in clusters, pairs and chains. Infectious disease was consulted. Antibiotic therapy was deescalated to intravenous (IV) ampicillin/sulbactam and a transthoracic echocardiogram was completed that did not reveal evidence of endocarditis. While awaiting microbe identification, the patient had an episode of bloody stool followed by a watery, non-bloody pellet-like stool. Stool studies from day 1 were negative for Shigella, Salmonella, Aeromonas, Clostridium difficile and Campylobacter.
On day 3, the organism on her blood cultures was identified. In both sets, the blood cultures grew E. cecorum. This organism was sensitive to ampicillin and vancomycin and synergistic with both streptomycin and gentamicin. Upon further questioning, there was no clear source of this organism as the patient denied interactions with poultry, domestic animals, recent travel, abnormal exposures, new skin lesions or changes to her diet. Repeat blood cultures drawn 48 hours after the initial 2 sets of blood cultures were drawn in the emergency department showed no growth. The patient was started on oral amoxicillin on day 4. The leukocytosis resolved, and she had had no further episodes of diarrhea, fevers or chills. She was discharged home on day 5. In total, she received 3 days of IV antibiotic therapy and was transitioned onto oral amoxicillin every 12 hours for 14 days on discharge.
Discussion
There are less than 10 total reported cases of E. cecorum infections in humans.2–7 This case report presents this typical poultry and domestic animal pathogen causing bloodstream infection in our human patient. Our patient’s diagnosis was first confounded due to her massive aortic aneurysm, history of gastric ulcer bleeding, SMA occlusion and coronary artery disease. Ischemic colitis was the presumed diagnosis due to her history of colitis and her vascular history. A colonoscopy was also not feasible. The patient was a poor candidate for endoscopy due to adverse effects with prior anesthesia and comorbidities. To our surprise, E. cecorum grew in both sets of blood cultures with negative stool cultures. We were fortunate that our patient improved with beta-lactam antibiotics initiated on admission, as many enterococci species are inherently resistant to many antibiotics, including penicillin.8
The purpose of this case is to bring awareness to a potential rare human pathogen of the Enterococcus genus. This bacterium’s human infectivity, the portal of entry, severity and effect on morbidity/mortality is unknown.
Conclusion
To the best of our knowledge, this case is the first reported since 2016 that reveals E. cecorum as a human pathogen, and it is the first to occur in the United States. Recording cases in the future involving this rare human pathogen is of utmost importance in order to shed light on its role in human disease processes.
Acknowledgements
The authors thank the patient for their informed consent.
Funding Statement
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity.
Footnotes
Conflicts of Interest
The authors declare they have no conflicts of interest.
The authors are employees of Regional Medical Center Bayonet Point, a hospital affiliated with the journal’s publisher.
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
References
- 1. Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology (Reading) 2009;155(Pt 6):1749–1757. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- 2. Greub G, Devriese LA, Pot B, Dominguez J, Bille J. Enterococcus cecorum septicemia in a malnourished adult patient. Eur J Clin Microbiol Infect Dis. 1997;16(8):594–598. doi: 10.1007/bf02447923. [DOI] [PubMed] [Google Scholar]
- 3. Delaunay E, Abat C, Rolain JM. Enterococcus cecorum human infection, France. New Microbes New Infect. 2015;7:50–51. doi: 10.1016/j.nmni.2015.06.004. Published 2015 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greub G, Devriese LA, Pot B, Dominguez J, Bille J. Enterococcus cecorum septicemia in a malnourished adult patient. Eur J Clin Microbiol Infect Dis. 1997;16(8):594–598. doi: 10.1007/bf02447923. [DOI] [PubMed] [Google Scholar]
- 5. De Baere T, Claeys G, Verschraegen G, et al. Continuous ambulatory peritoneal dialysis peritonitis due to Enterococcus cecorum. J Clin Microbiol. 2000;38(9):3511–3512. doi: 10.1128/jcm.38.9.3511-3512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woo PC, Tam DM, Lau SK, Fung AM, Yuen KY. Enterococcus cecorum empyema thoracis successfully treated with cefotaxime. J Clin Microbiol. 2004;42(2):919–922. doi: 10.1128/jcm.42.2.919-922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warnke P, Köller T, Stoll P, Podbielski A. Nosocomial infection due to Enterococcus cecorum identified by MALDI-TOF MS and Vitek 2 from a blood culture of a septic patient. Eur J Microbiol Immunol (Bp) 2015;5(2):177–179. doi: 10.1556/1886.2015.00008. Published 2015 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmed FZ, Baig MW, Gascoyne-Binzi D, Sandoe JA. Enterococcus cecorum aortic valve endocarditis. Diagn Microbiol Infect Dis. 2011;70(4):525–527. doi: 10.1016/j.diagmicrobio.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Kristich CJ, Rice LB, Arias CA. Enterococcal Infection—Treatment and Antibiotic Resistance. In: Gilmore MS, Clewell DB, Ike Y, et al., editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection [Internet] Boston: Massachusetts Eye and Ear Infirmary; 2014. https://www.ncbi.nlm.nih.gov/books/NBK190420/ [PubMed] [Google Scholar]