Figure 1.
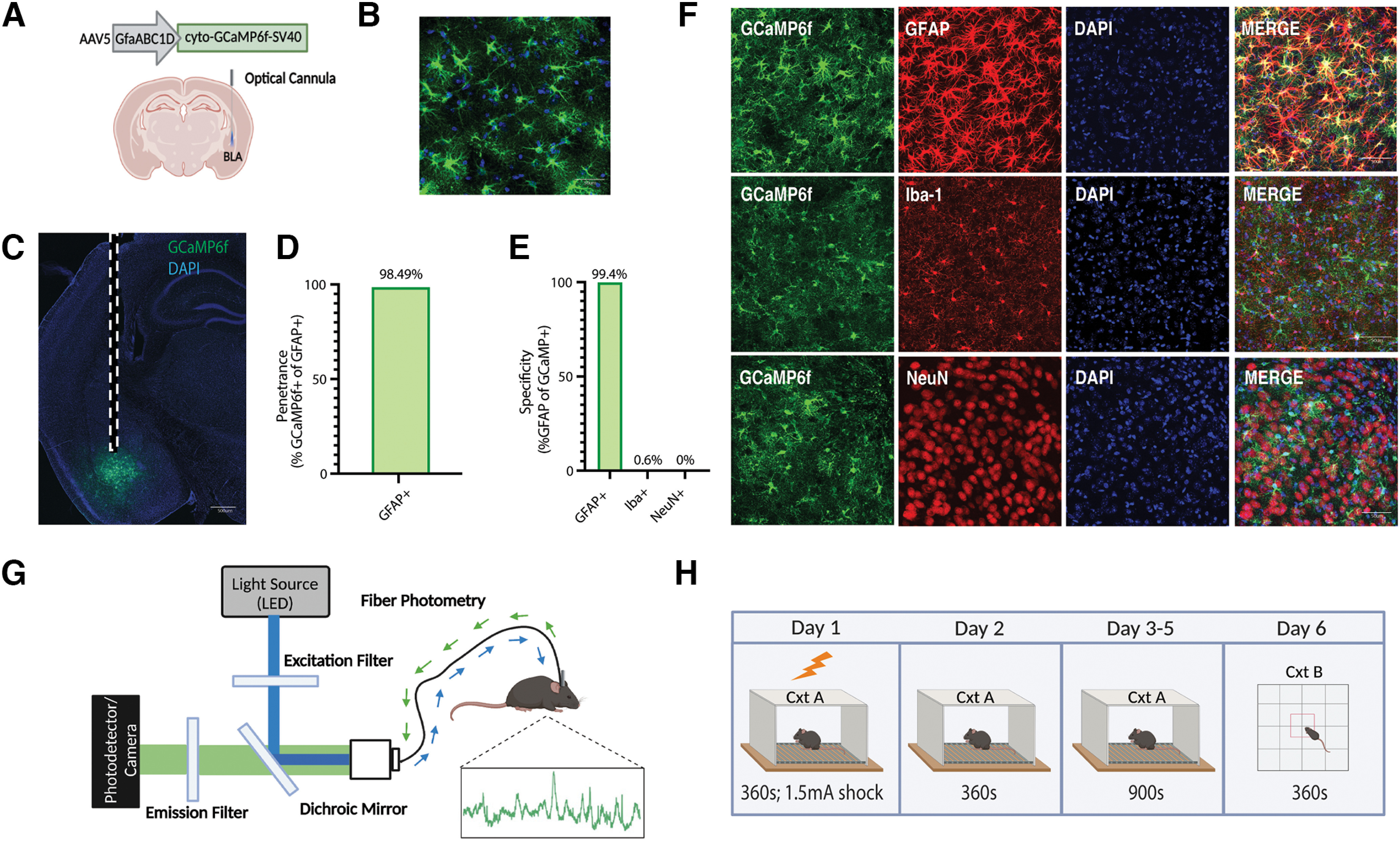
Population-level calcium recordings of basolateral amygdala astrocytes across contextual fear conditioning, recall, and extinction. A, Viral strategy and fiber implantation strategy for shock and no-shock conditions. The GECI AAV5-GfaABC1D-cyto-GCaMP6f-SV40 was unilaterally injected into the BLA region of wild-type mice. B, C, Representative image of GFAP-GCaMP6f+ (green) and DAPI+ (blue) cell expression within the BLA at 20× magnification (B) and 10× magnification (C). Scale bar, 500 μm. Dashed white lines indicate the approximate location of the unilateral fiber implantation. D, Penetrance of GCaMP6f (2251 GCaMP6f+/2381 GFAP+ = 98.49%; n = 3; 4 slices/mouse). E, Specificity of GCaMP6f (4 Iba-1+/662 GCaMP6f+ = 0.604% microglia; 0 NeuN+/1064 GCaMP6f+ = 0.00% neurons; 2242 GFAP+/2256 GCaMP6f+ = 99.4% astrocyte; n = 3; 4 slices/mouse). F, Representative expression of GCaMP6f expression and overlap with microglial (Iba-1), astrocytic (GFAP), and neuronal (NeuN) markers. Scale bar, 50 μm. G, In vivo fiber photometry setup. A 470 nm LED delivered an excitation wavelength to GCaMP6f-expressing astrocytes via a patch cord and single fiber optic implant in freely moving mice. The emitted 530 nm signal from the indicator was collected via the same patch cord and fiber, spectrally separated using a dichroic mirror, passed through a series of filters and focused on a scientific camera. A representative calcium time series trace is shown for astrocytic calcium. Calcium-independent isosbestic signal was recorded simultaneously to account for motion, tissue autofluorescence, and photobleaching across time. H, Behavioral paradigm; mice underwent CFC on day 1 in Cxt A for 360 s where they received four 1.5 mA foot shocks. On day 2, mice were placed back into Cxt A for contextual recall for 360 s in the absence of foot shock. On days 3–5, mice underwent three contextual extinction sessions for 900 s each. On day 6, mice were placed in Cxt B for 360 s. Mice were perfused and brains extracted for histologic assessment.
