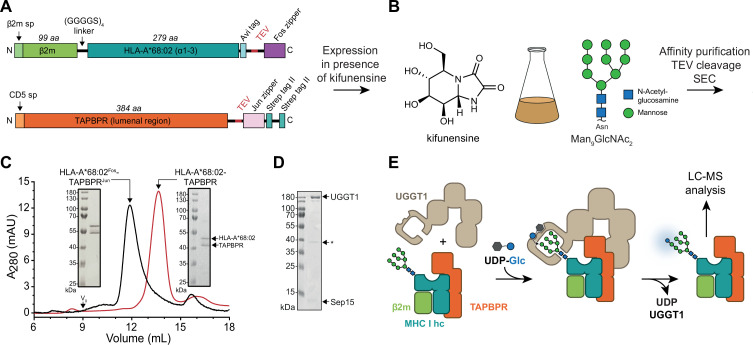
Figure 1. In-vitro system of UGGT1-catalyzed quality control and reglucosylation of MHC I.
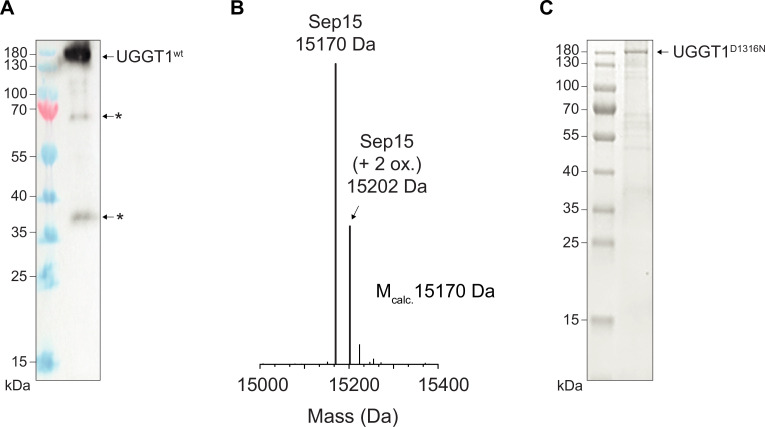
(A) Construct design to obtain peptide-receptive HLA-A*68:02-TAPBPR complexes by transient co-transfection of HEK293-F cells. (B) Co-expression of HLA-A*68:02 and TAPBPR was performed in the presence of the α-mannosidase I inhibitor kifunensine to generate a defined Man9GlcNAc2 glycan tree on the MHC I that can be recognized by UGGT1. (C) The secreted leucine zippered complex was isolated from the cell culture supernatant by Strep-Tactin affinity purification. Leucine zippers and the Twin-Strep-tag of TAPBPR were removed by protease treatment. The MHC I-chaperone complexes were further analyzed and purified by size exclusion chromatography (SEC) on a Superdex 200 (Increase 10/300) column. (D) SDS-PAGE analysis of immobilized metal-affinity chromatography (IMAC)-purified human wildtype (wt) UGGT1 co-expressed with Sep15 and secreted from insect cells. The asterisk (*) indicates a degradation product of UGGT1. (E) The purified UGGT1-Sep15 complex was employed in the reglucosylation assay with the peptide-receptive HLA-A*68:02-TAPBPR complex of (C) harboring the high-mannose glycan on MHC I. Abbreviations: A280: absorption at 280 nm; aa: amino acids; kDa: kilodalton; mAU: milli-absorption units; MHC I hc: MHC I heavy chain; sp: signal peptide; UDP-Glc: UDP-glucose; V0: void volume.
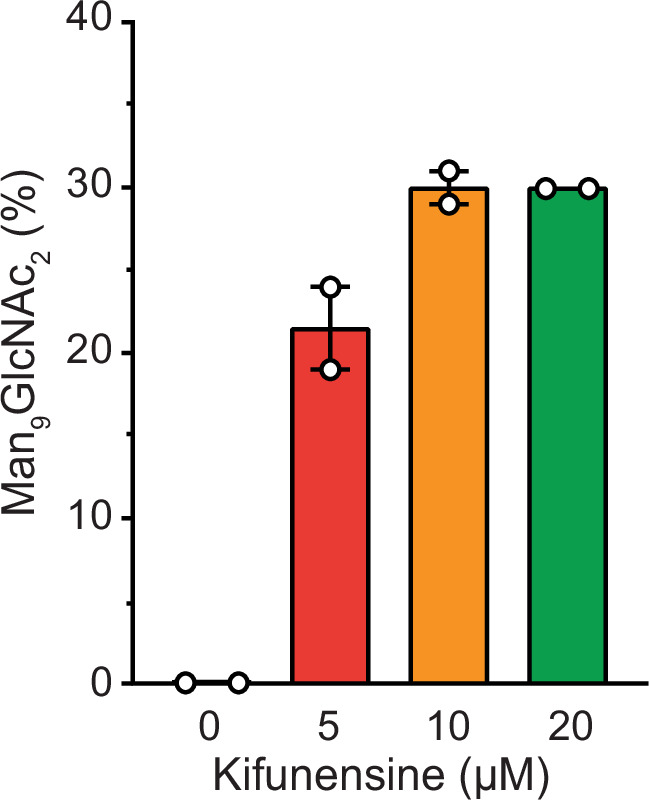
Figure 1—figure supplement 1. Influence of kifunensine concentration on the proportion of Man9GlcNAc2 among the Man7-9GlcNAc2 MHC I glycan species when co-expressed with TAPBPR in HEK293-F cells.