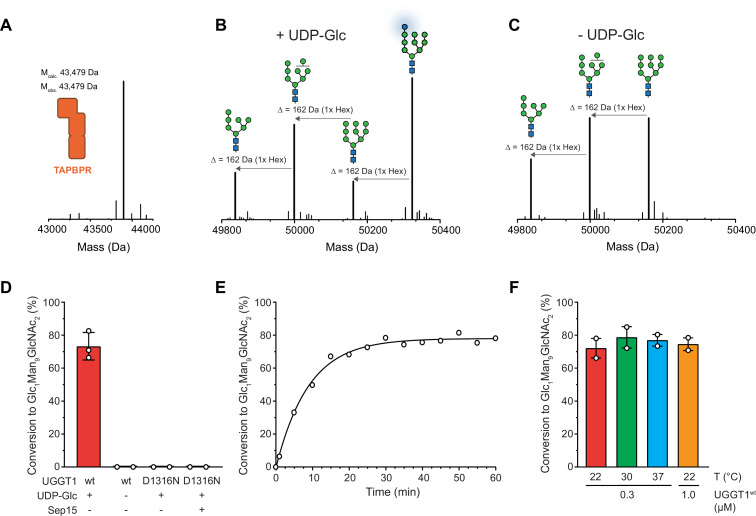
Figure 2. UGGT1 catalyzes reglucosylation of TAPBPR-bound HLA-A*68:02.
Protein identities in the reconstituted glucosylation system and the glycan status of HLA-A*68:02 (Man7-9GlcNAc2 and Glc1Man9GlcNAc2) during the UGGT1-catalyzed reglucosylation reaction were determined by liquid chromatography-mass spectrometry (LC-MS). (A) Deconvoluted mass spectrum of TAPBPR. (B) HLA-A*68:02-TAPBPR (3 µM) complex was incubated with UGGT1 (1 µM) in the absence (-) and (C) presence (+) of UDP-glucose (UDP-Glc) for 1 h at room temperature. In the presence of UDP-Glc, UGGT1 catalyzed the glucose transfer, generating the mono-glucosylated glycan (Glc1Man9GlcNAc2), whereas the native glycan pattern remained unchanged in the absence of UDP-Glc. Exemplary deconvoluted mass spectra of glycosylated HLA-A*68:02 representing LC-MS analyses summarized in (D) are shown. (D) Comparison of glucosyltransferase activity, as measured by the amount of produced Glc1Man9GlcNAc2-HLA-A*68:02, upon incubating HLA-A*68:02-TAPBPR (3 µM) with UGGT1 proteins (1 µM) in the absence (-) and presence (+) of UDP-Glc and additional purified Sep15 (5 µM), respectively. Data represent mean ± SD (n=3) and (n=2), respectively. (E) Kinetics of reglucosylation of HLA-A*68:02-TAPBPR (3 µM) catalyzed by UGGT1 (1 µM). (F) Temperature dependence of UGGT1-catalyzed reglucosylation of HLA-A*68:02-TAPBPR (3 µM). Reactions were stopped after 60 min. Data represent mean ± SEM (n=2). Abbreviations: Da: dalton; Glc: glucose; GlcNAc: N-acetylglucosamine; Hex: hexose; Man: mannose; Mcalc: calculated mass; Mobs: observed mass.
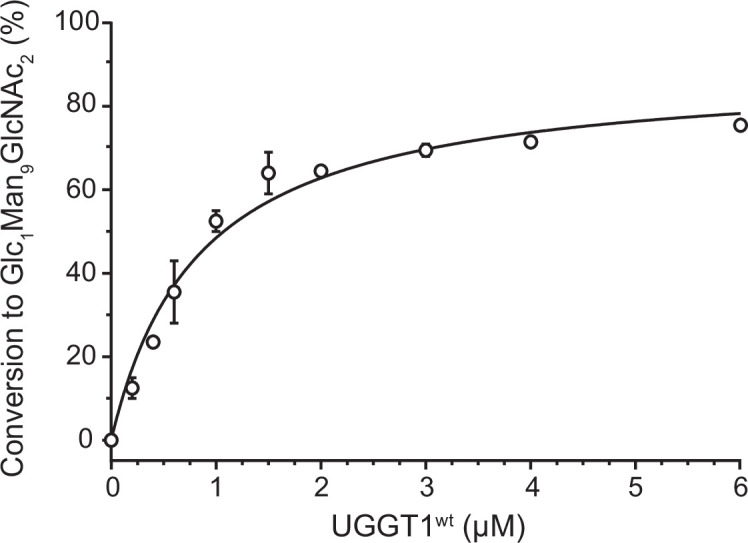
Figure 2—figure supplement 1. UGGT1 concentration-dependent reglucoslyation kinetics of TAPBPR-bound HLA-A*68:02.