Figure 1.
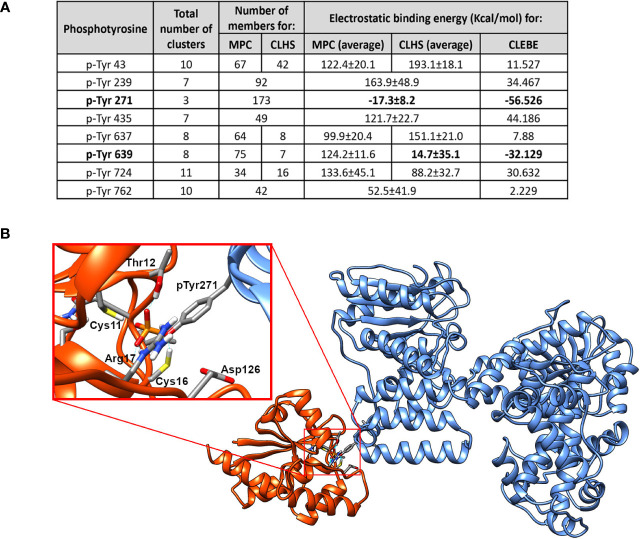
The P-Tyr271 of hTFPα is the potential target of mycobacterial PtpA. (A) Table showing the results of the docking assays between mycobacterial PtpA and hTFPα. The total number of clusters obtained for each p-Tyr evaluated, the number of members, and the electrostatic binding energy are indicated. MPC: Most Populated Cluster; CLHS: Cluster with the Lowest Haddock Score, CLEBE: Complex with the Lowest Electrostatic Binding Energy. In the case where the MPC is the same as the CLHS, both columns are merged into one. (B) Molecular structure of the best model of the PtpA/hTFPα complex obtained after docking assays. In the enlarged inset, the most critical residues of the PtpA (orange) binding region and hTFPα (blue) are represented as sticks. These include the catalytic residues of PtpA (Cys11, Thr12, Cys16, Arg17, Asp126) and the p-Tyr 271 of hTFPα.