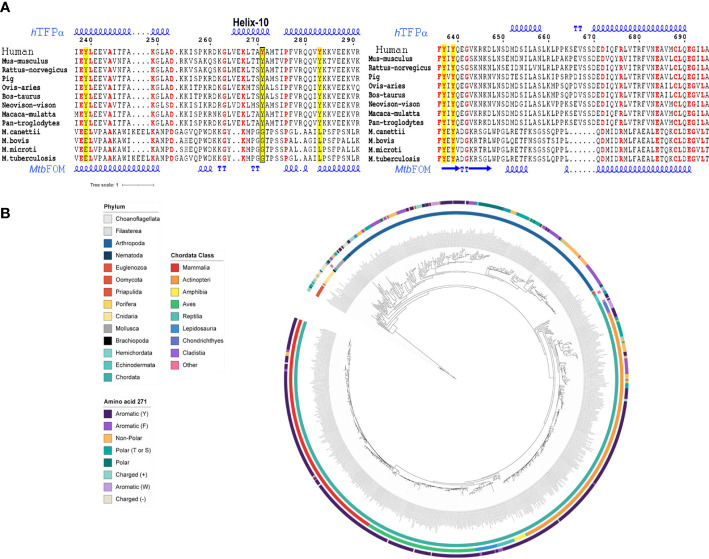
Figure 2.
The Tyr271 of hTFPα is conserved in several eukaryotic protein homologs and part of the helix-10, absent in bacterial homologs. (A) Multiple sequence alignment of hTFPα homologous, representative of mammals and bacterial TB complex. The Tyr271 (left alignment) and Tyr639 (right alignment) conservation are shown. The complete alignments are shown in Figures S1 – S3 . The tyrosines of hTFPα are in red and highlighted in yellow. The box indicates the Y271 residue placed in the helix-10 of the hTFPα. The alignment was obtained using the MUSCLE server (Edgar, 2004), and the figure was done using the ESPrit 3.0 server (Robert and Gouet, 2014). The secondary protein structure of hTFPα and the Mtb FOM are also shown, based on the pdb structures 6DV2 (Xia et al., 2019) and 4B3H (Venkatesan and Wierenga, 2013), respectively. (B) Eukaryotic TFPα phylogenetic analysis. Circular representation of a maximum likelihood tree of TFPα of eukaryotes ranging from euglenozoos to vertebrates. The tree is midpoint rooted. Inner circle: phylum colored according to the legend. Middle circle: Chordata class colored according to the legend. Outer circle: amino acid present in each gene at the position aligned to human Tyr-271, colored according to the legend. For details, see Figure S3 .