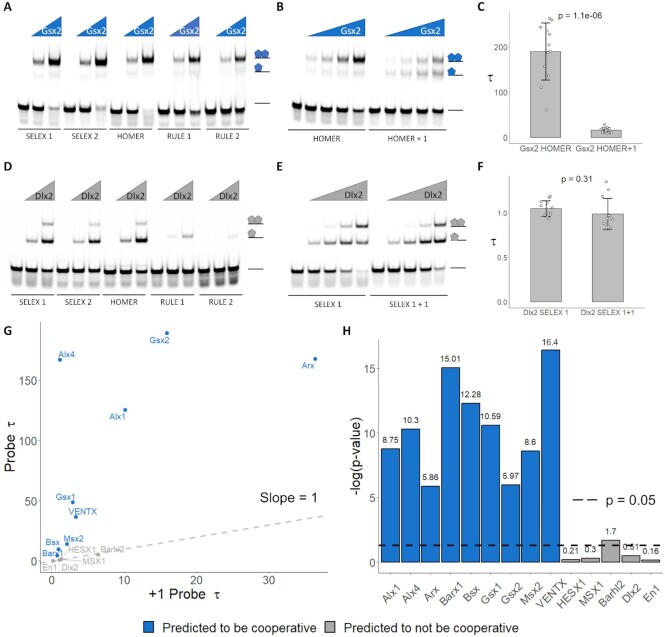
Figure 3.
Spacer-dependent cooperativity systematically assessed via EMSAs. (A, D) The binding activity of five fluorescently labeled probes (34 nM) to two Gsx2 protein concentrations (A; 100 and 400 nM) or Dlx2 protein concentrations (D; 20 and 80 nM) was determined using EMSAs. Schematics at right represent protein-DNA complexes. Note, Gsx2 preferentially binds all probes as a dimer whereas Dlx2 preferentially binds all probes as a monomer. (B, E) The highest affinity probe for Gsx2 (HOMER) and Dlx2 (SELEX 1) were subsequently used to calculate a cooperativity factor for each TF. To assess for spacer length dependence, we also tested Gsx2 on the HOMER + 1 and Dlx2 on the SELEX 1 + 1 probes in which a single additional bp was added between the 4mers. Each EMSA consisted of 30 lanes in which the 2 probes were tested at 4 concentrations in triplicate. A single replicate is shown here, and all replicates are shown in Supplementary Figure S7. 34 nM of probe was used in each lane and the protein concentrations tested were as follows (B; Gsx2 = 0, 31.25, 62.5, 125, and 250 nM) and (E; Dlx2 = 0, 20, 40, 80 and 160 nM). Schematics of the protein-DNA complexes are shown to the right of each gel. (C, F) Tau cooperativity factors were calculated for each binding reaction in which the TF was added. Bar graphs depict the average Tau factor for each TF with each dot representing a Tau factor from an individual binding reaction (n = 12 for each group). Error bars denote standard deviation. Tau cooperativity factors were compared with two-sided unpaired student t-tests. Note, Gsx2 has a Tau of 189 on the Homer probe, indicating that the binding of the first site facilitates the binding of the second site 189-fold. However, the addition of the nucleotide to the spacer disrupted cooperativity and caused a significant decrease in Tau. In contrast, Dlx2 has a Tau factor of ∼1 on both probes, indicating that the binding of the first site has no impact on the binding of the second site to either probe. (G) This approach was applied to 9 TFs that were predicted to bind cooperatively (blue text) and 5 TFs that were not predicted to bind cooperatively (gray text). TFs that were predicted to bind cooperatively had significantly higher Tau factors on their respective high affinity probes (Probe Tau) compared to the probes in which a single bp was added between the two sites (+1 Probe Tau), demonstrating spacer dependent cooperativity. Conversely, TFs that were not predicted to bind cooperatively had similar Tau values between the two probes. Data points that fall on the gray dashed line have equal Tau factors on both probes. (H) Bar graph comparing the log10P-values of the Tau values for the high affinity compared to the +1 probes for each TF. P-values that are higher than the dashed line indicate significance. TFs predicted to bind cooperatively had significantly different cooperativities between the two probes, whereas predicted non-cooperative TFs did not with the exception of Barhl2. Note, however, that Barhl2 was weakly cooperative to both probes and had a significantly higher Tau factor for its +1 probe compared to its high affinity probe, demonstrating spacer independent cooperativity.