Abstract
The precise activation of C–H bonds will eventually provide chemists with transformative methods to access complex molecular architectures. Current approaches to selective C–H activation relying on directing groups are effective for the generation of five-membered, six-membered and even larger ring metallacycles but show narrow applicability to generate three- and four-membered rings bearing high ring strain. Furthermore, the identification of distinct small intermediates remains unsolved. Here, we developed a strategy to control the size of strained metallacycles in the rhodium-catalysed C−H activation of aza-arenes and applied this discovery to tunably incorporate the alkynes into their azine and benzene skeletons. By merging the rhodium catalyst with a bipyridine-type ligand, a three-membered metallacycle was obtained in the catalytic cycle, while utilizing an NHC ligand favours the generation of the four-membered metallacycle. The generality of this method was demonstrated with a range of aza-arenes, such as quinoline, benzo[f]quinolone, phenanthridine, 4,7-phenanthroline, 1,7-phenanthroline and acridine. Mechanistic studies revealed the origin of the ligand-controlled regiodivergence in the strained metallacycles.
Subject terms: Homogeneous catalysis, Synthetic chemistry methodology, Density functional theory
The ability to selectively functionalize different sites on simple starting materials is a constant pursuit in organic chemistry. Here, the authors report a catalytic system to regioselectively differentiate and alkynylate different positions on azaarenes via rhodium catalysis.
Introduction
Due to the near universal advantage of C − H bonds in organic molecules, the C − H activation strategy provides an opportunity to functionalize any carbon centre in an atom-economical and streamlined way1–10. Because organic molecules typically contain multiple C − H bonds with comparable strengths and steric environments, regiocontrol has been a long-standing challenge within this type of chemistry11. The differentiation of C − H bonds is traditionally dominated by steric and electronic effects12, and there have been considerable efforts to utilize directing groups in less constrained molecules (Fig. 1a)13–17. Directed C − H activation is thermodynamically favoured through conformationally rigid five-, six-, and seven-membered metallacyclic intermediates. Several elegant methods have been exploited to achieve the meta- and para-selective C − H activation of arenes through chelation-assisted macrocyclic complexes by directing groups18–22. Despite these advances, the generation of highly strained metallacycles in directed C − H activation remains much desired but more challenging.
Fig. 1. Background and discovery.
a General procedures for selective C–H activation assisted by directing groups. b α-Selective C–H functionalization of aliphatic amines via metallaaziridine intermediates. c β-Selective C–H functionalization of aliphatic amines via four-membered metallacycles. d Modular differentiation of quinolines by tunable three- and four-membered ring cyclometallation.
Substantial progress has been made regarding the C–H activation of aliphatic amines through strained metallacycles, allowing for site-selective functionalization at the α or β position of the amino group in one catalytic step23,24. Early transition metals, including titanium, tantalum and zirconium, enable the hydroaminomethylation of alkenes with unprotected N-heterocycles and amines through metallaaziridine intermediates, affording either linear or branched products (Fig. 1b)25–30. A series of C–H functionalizations of aliphatic amines have been developed by palladium catalysis, showing β-selectivity through a four-membered ring cyclopalladation pathway (Fig. 1c)31–33. Compared to small aliphatic metallacycles, the formation of benzo-fused analogues comes with larger ring strain. We questioned whether such metallacycles could be generated through chelation with inherent nitrogen atoms in aza-arenes. Furthermore, switching the ring size in a unifying system to differentiate of two C–H bonds would likely have a broad impact on the continued advancement of this field.
As a representative example of bicyclic aza-arenes, quinoline contains seven C − H bonds in the pyridine (C2 to C4) and benzene (C5 to C8) cores that can be functionalized34,35. Structural modification of the pyridine ring has been achieved by taking advantage of strong electronic and steric bias36–43. An indirect route to access the benzene core involves the oxidation of quinoline to its N-oxide, followed by oxygen-directed C − H functionalization and subsequent reduction to the desired products44–46. To provide more flexibility to access the benzene core, metal clusters and dimers such as Os3(CO)1047, Ru3(CO)1248, and Rh2(OAc)449 have been employed in C − H activation through bridged metal-metal bonds. Enabled by bimetallic palladium catalysis, Yu and coworkers also designed a class of remote-directing template for differentiation of C − H bonds (C5-C7) at the benzene core of quinolines50–53.
Here, we report the successful realization of a strategy for regiodivergent C–H activation of aza-arenes enabled by tunably strained metallacycles under monomeric rhodium catalysts (Fig. 1d). The precise differentiation of two C–H bonds proceeds through a switchable three- or four-membered-ring cyclometallation pathway by just tuning the structure of the ligands. Incorporation of alkyne motifs into aza-arenes are valuable transformations to build C–C bonds and provide a versatile handle for further modifications.
Results
Reaction design
As shown in Fig. 2, we first evaluated the reaction of 3-methylquinoline (1a) with (bromoethynyl)triisopropylsilane (2a). After treatment of [Rh(cod)Cl]2 (5 mol%) with NaOtBu (2.5 equiv) at 120 °C under an Ar atmosphere in toluene, the desired product 3aa was generated in trace amounts after 12 h (entry 1). After screening a variety of ligands for optimization (for the details of the reaction optimization, see the Supplementary Information), the optimized conditions for C2-alkynylation were determined, using 10 mol% dtbpy as a ligand. Under these conditions, product 3aa was afforded in 84% yield, showing 99/1 r.r. of the C2 to C8 positions (entry 2). Interestingly, employing NHC ligands54 resulted in a preference for C8 selectivity, and the use of IMes·HCl led to the formation of product 4aa in 70% yield (C8/C2 = 91/9 r.r.) (entry 3). Employment of [Rh(cod)Cl]2 with the optimal ligands resulted in the production of two rhodium complexes, Rh(cod)(dtbpy)Cl and Rh(cod)(IMes)Cl, both of which were unambiguously assigned by X-ray crystallography. Utilizing the pregenerated Rh(cod)(dtbpy)Cl as the catalyst showed a much lower reactivity than the in situ generation of the catalyst (entry 4). In contrast, the use of Rh(cod)(IMes)Cl as the catalyst improved the generation of 4aa to 85% yield with 92/8 r.r. (entry 5).
Fig. 2. Effect of the ligands on reactivity and regioselectivity.
The regioselectivity ratio (r.r.) was determined by GC‒MS analysis of the reaction mixtures. dtbpy = 4,4’-di-tert-butyl-2,2’-bipyridine. IMes =1,3-bis (2,4,6-trimethylphenyl)−1,3-dihydro-2H-imidazol-2-ylidene.
Scope of the methodology
The scope of the regiodivergent C–H alkynylation was then examined (Fig. 3). In general, a series of commercially available quinolines with varied substituents reacted smoothly with bromoalkyne 2a, yielding two types of alkynylation products under reaction conditions A and B. The reaction of quinoline (1b) with bromoalkyne 2a underwent C2- and C8-selective C–H alkynylation, providing products 3ba (98/2 r.r.) and 4ba (89/11 r.r.) with excellent regioselectivities. The quinolines that incorporated electron-donating groups, including phenyl (1c), ether (1d-e), silyloxy (1 f), and amino (1g-h) groups, at different positions were readily tolerated with bromoalkyne 2a to produce the corresponding products 3ca-ha and 4ca-ha with excellent regioselectivity under both reaction conditions. Halide-containing quinolines 1i-p bearing F, Cl, Br, and even I motifs were compatible with the generation of both the C2 and C8 alkynylation products. Among them, substrates 1k-l containing substituents at the C7 position did not sterically block the reaction at the C8 position. Quinoline 1q bearing a CF3 group was also compatible with both sets of reaction conditions, affording 3qa and 4qa in 87% and 64% yields, respectively. The use of 6-cyanoquinoline (1r) under condition A became too sluggish to provide product 3ra, but this substrate did not interfere with productive C–H alkynylation at the C8 position. Quinoline 1 s, containing a phenylethynyl motif at the C3 position inhibited the reactivity at the C2 position but enabled C8-selective C–H alkynylation to generate product 4sa. As a bidentate nitrogen ligand, 2,2′-biquinoline (1t) could also engage under reaction conditions B to deliver the expected products 4ta in a good yield. We further explored the scope of alkynyl bromides with quinoline 1a. Compared to reagent 2a, the reaction of TIPS-protected ethynyl chloride (2a’) showed much lower reactivity in both reaction systems. Alkynylation with propargyl silyl ethers 2b-c afforded the desired products in significantly lower yields, but maintained the excellent regioselectivity. Alkynyl bromides 2d with a less sterically hindered phenyl group were problematic under both reaction conditions, and the corresponding products 3ad and 4ad were observed in only trace amounts. The poor outcomes are attributed to the lower stability of these bromoalkynes without TIPs group under the reaction conditions55–59.
Fig. 3. Substrate scope of the regiodivergent C–H alkynylation of quinolines at the C2 and C8 positions.
Reaction Conditions A: [Rh(cod)Cl]2 (5 mol%), dtbpy (10 mol%), 1 (0.20 mmol, 1.0 equiv), 2 (0.50 mmol, 2.5 equiv), NaOtBu (0.50 mmol, 2.5 equiv), 1 mL of toluene, 120 °C, 12 h, under argon. Reaction Conditions B: [Rh(cod)(IMes)Cl (10 mol%), 1 (0.20 mmol, 1.0 equiv), 2 (0.50 mmol, 2.5 equiv), NaOtBu (0.50 mmol, 2.5 equiv), 1 mL of toluene, 120 °C, 12 h, under argon. r.r. values were determined by GC‒MS analysis of the reaction mixture.
Other aza-arenes commonly featured in bioactive molecules and functional materials were next targeted. Benzo[f]quinoline (5a) was first employed in the catalytic systems and gave desired products 6aa and 7aa at the C3 and C5 positions, respectively (Fig. 4a). Phenanthridine (5b), the key aromatic unit of many DNA stains, underwent regiodivergent C–H alkynylation at the C6 and C4 positions in high yields with excellent selectivities (Fig. 4b). Treatment of 4,7-phenanthroline (5c) containing two azine motifs under reaction conditions A afforded a promising mixture of mono- and di-substituted products 6ca and
Fig. 4. Regiodivergent C–H alkynylation of diverse aza-arenes.
a C–H alkynylation of benzo[f]quinolone (5a). b C–H alkynylation of phenanthridine (5b). c C–H Alkynylation of 4,7-phenanthroline (5c). d C–H alkynylation of 1,7-phenanthroline (5d). e C–H alkynylation of acridine (5e).
6ca’ with a high regioselectivity (Fig. 4c). Similarly, the two products 7ca and 7ca’ were also generated under reaction conditions B with excellent regioselectivity. Notably, the products with one or two alkynyl motifs could be separated by column chromatography on silica. When 1,7-phenanthroline (5d) was treated under reaction conditions A, C–H alkynylation occurred at the C8 position. However, an unusual C10-functionalized product 7da’ was obtained under reaction conditions B, indicating that C–H metalation process preferred to undergo five-membered metallacyclic intermediate (Fig. 4d). Finally, acridine (5e), bearing two C–H bonds (C4 and C5) for activation, gave only the monosubstituted product 7ea in 85% yield with complete selectivity (Fig. 4e).
Synthetic applications
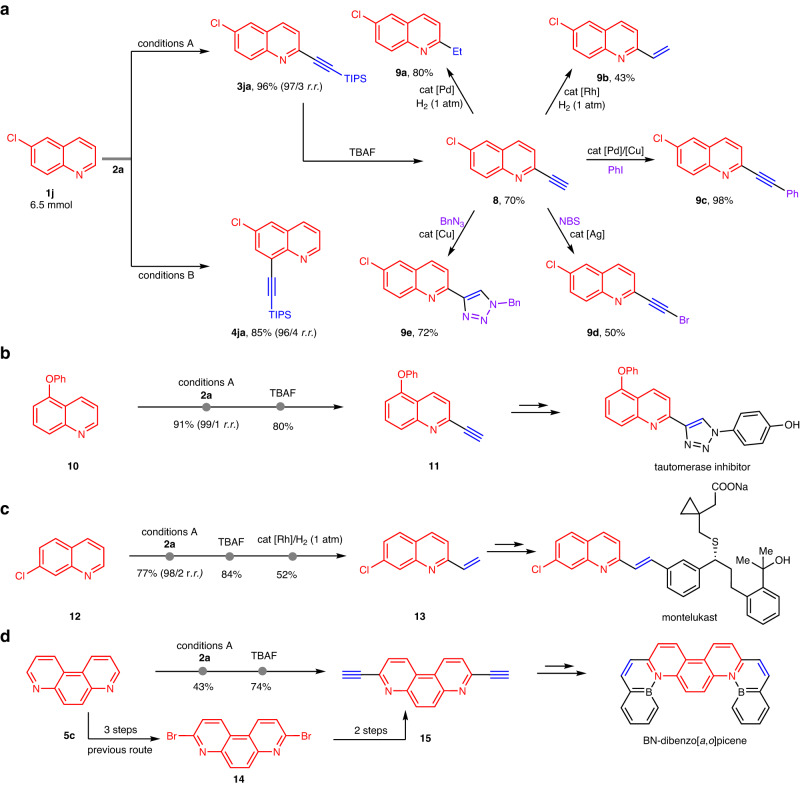
To showcase the synthetic utility of this discovery, gram-scale reactions of quinolone 1j were first performed under both reaction conditions, and products 3ja and 4ja were isolated in 96% and 85% yields without erosion of the regioselectivity (Fig. 5a). Further derivatizations of 3ja were achieved with synthetically useful intermediate 8, which was obtained in 70% yield by the TBAF-mediated removal of the TIPS group. For instance, hydrogenation of 8 under 1 atm of hydrogen with a catalytic amount of Pd/BaCO3 yielded alkylation product 9a in high yield. The use of the Wilkinson catalyst for hydrogenation allowed for the chemoselective synthesis of olefin 9b. The terminal triple bond in 8 could be effectively transformed into an internal bond to provide product 9c in nearly quantitative conversion through the Sonogashira reaction. Ag catalysis of the reaction of 8 with NBS formed bromination product 9d in excellent yield. In addition, alkyne 8 was easily converted to triazole 9e with BnN3 via a copper-catalysed click reaction. Based on diverse alkyne conversion, our strategy provides a simple and distinct way to construct a number of pharmaceutically relevant compounds and materials. The reaction of quinoline 10 under Conditions B furnished C2-alkynylation, and further removal of the TIPS group provided Compound 11 in good yield, acting as a core framework for the tautomerase inhibitor (Fig. 5b). As a key intermediate for the synthesis of montelukast, Compound 13 was rapidly prepared from quinoline 12 by C2-selective C–H alkynylation with bromoalkyne 2a, further removal of the TIPS group and reduction with the Wilkinson catalyst under 1 atm of hydrogen (Fig. 5c). Compound 15 is a key intermediate in the synthesis of BN-dibenzo[a,o]picene, and 3,8-dibromo-4,7-phenanthroline (14) must be pregenerated through multistep synthesis from 5c to access this molecule by Sonogashira coupling (Fig. 5d)60. Notably, this molecule can be prepared in an atom- and step-economic way, further indicating the value of the developed method.
Fig. 5. Synthetic applications of regiodivergent C–H alkynylation.
a Gram-scale preparation of 3ja and 4ja and their derivatizations. b Application of the process to the synthesis of potent tautomerase inhibitors. c Application of the process to the synthesis of montelukast. d Application of the process to the synthesis of BN-dibenzo[a,o]picene.
Mechanistic study
In order to gain insights into the reaction pathways and origin of positional selectivity, density functional theory (DFT) calculations were performed using the reaction of quinoline 1b and alkynyl bromide 2a as the model (Fig. 6). The reaction initially commences by the formation of 1b and the ligand bound Rh(I) intermediate INT1A, which is set as the relative zero point of the Gibbs free energy. The previous reported Rh(I)-involved C–H activation by oxidative addition might be a reversible process with an activation energy barrier of 26.2 kcal mol−1 (for the details of the computational data, see the Supplementary Information)36–38. Comparably, INT1A more favorably coordinates with 2a to generate INT2A, which then undergoes C–Br bond oxidative addition to afford Rh(III) species INT3A. In the case of the dtbpy ligand, the C–Br oxidative addition through transition state TS3A-dtbpy was calculated to have an activation free energy of 27.6 kcal mol−1. This step is the rate-determining step in the catalytic cycle. The resulting intermediate INT3A-dtbpy occurs C2–H metalation through a tbutoxide-assisted deprotonation with a 25.8 kcal mol−1 energy barrier61–66. Further reductive elimination and ligand exchange yield the desired products with high selectivity and regenerates INT1A to complete the catalytic cycle. For the IMes ligand, the C–Br bond oxidative addition to the Rh(I) center from INT2A-IMes has an activation energy of 26.7 kcal mol−1, which shows a comparable energy barrier with the subsequent C–H activation (26.1 kcal mol−1). In the following C − H metalation, IMes ligand favors C8 selective transition state TS4B-IMes.
Fig. 6. Calculated energy profiles of the regiodivergent C–H alkynylation of 1b with 2a.
a The proposed catalytic cycles with different ligands. b The key transition states in the catalytic cycle.
As shown in Fig. 7, extensive computational studies on different site selectivity were also carried out. The molecular electrostatic potential map was used to analysis the possible nucleophilic sites of quinoline 1b (Fig. 7a). Quantitatively, the natural population analysis of 1a shows that C2 has an atomic charge of −0.069e, which is less charged than C8 (−0.192e), illustrating C2 − H is relative electron deficient and more prone to C − H metallization with electron-rich rhodium catalyst (Supplementary Data 1). Inspecting computed electrostatic potential maps of Rh−dtbpy and Rh−IMes, the dtbpy ligand exhibits more electron-donating ability than IMes, allowing INT3A-dtbpy to preferentially react with the C2 position of 1b. The energy barrier of the rhodacycle transition state TS4A-dtbpy that delivers the three-membered intermediate INT4A-dtbpy is 8.2 kcal mol−1 lower than that of the competing transition state TS4B-dtbpy that leads to the INT4B-dtbpy (Fig. 7b). This energy difference is consistent with the experimentally excellent site selectivity of 3ba. Without the nitrogen chelation, transition states TS4C-dtbpy and TS4D-dtbpy have activation barriers of 42.0 and 43.9 kcal mol−1, respectively. Such high computed energies are mainly ascribed to the intrinsic inertness of the C − H bonds at the C2 and C8 position of quinoline, indirectly illustrating the importance of the directing group. The directed C–H metalation transition state through TS4B-IMes has an activation Gibbs free energy of 26.1 kcal mol−1, which is 17.3 kcal mol−1 lower than the same process through TS4A-IMes (26.1 and 43.4 kcal mol−1) and 11.2 kcal mol−1 lower than the original nucleophilic attack at quinoline C2 position through TS4C-IMes (26.1 and 37.3 kcal mol−1). In addition, the steric maps around the Rh(III) centre with IMes ligand were analyzed based on the SambVca 2.1 tool (Fig. 7c)65,66. All transition states adopt trigonal bipyramidal geometries, where Br atom and OtBu group occupy the two vertex positions and the IMes ligand in the NW quadrant of the steric map extends to the NE and SW quadrants. In disfavored transition state TS4A-IMes, bicyclo 3-4 fused rhodacycle enables the Rh−C bond formation to occur in the SW quadrant, which suffers from significant repulsion with Mes group of the carbene ligand. The favored transition state TS4B-IMes is a bicyclo 4-4 fused rhodacycle, the expansion of ring size makes the quinoline backbone more horizontally extended and reduce the steric hindrance between quinoline and TIPS group, thereby effectively lowering the energy barrier of TS4B-IMes. In TS4C-IMes and TS4D-IMes, Rh−N bond distances are elongated to 2.85 and 3.57 Å, respectively, indicating nitrogen atom in 1b is not anchored around the Rh(III) centre. Taken together, the electronic and steric effects account for the tunability of the strained metallacycles, achieving different positional selectivity67,68.
Fig. 7. The origin of the site selectivity.
a Molecular electrostatic potential map of 1b, Rh−dtbpy, and Rh−IMes. b Key transition states for the different site selectivities. c Steric maps of the Rh(III) transition states TS4A-IMes, TS4B-IMes, TS4C-IMes, and TS4D-IMes.
Discussion
In conclusion, the present results demonstrate an important initial advance in C–H activation through a benzo-fused three- or four-membered ring cyclometallation pathway in a switchable mode. This chemistry provides a unique tool for the functionalization of high-value aza-arenes with divergent site selectivities controlled by ligands. The switch of the positional selectivity through strained metallacycles fills a major methodological gap in directed C–H activation. We anticipate that other transformations based on this strategy could be exploited for molecular editing of aza-arene C–H bonds, providing inspiration for the design of new tactics to produce complex bioactive molecules, natural products and functional materials.
Methods
Due to slight variations in experimental protocols for all the processes we present, we refer the reader to the Supplementary Methods for experimental details.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We would like to thank the National Natural Science Foundation of China (Grants 22025104, 22171134, 21972064, and 21901111), the Fundamental Research Funds for the Central Universities (Grant 020514380254) for their financial support and Tang Scholar. We are also grateful to the High-Performance Computing Center of Nanjing University for performing the numerical calculations in this paper on its blade cluster system.
Author contributions
L.X. developed the catalysts and reactions and performed and analyzed experiments. M.W. performed the DFT calculation. Y.L. supervised the DFT calculation and commented on the manuscript. Y.Z. performed the crystallographic studies; Z.S. conceived and supervised the project and wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
Crystallographic data for the structures of Rh(cod)(dtbpy)Cl, Rh(cod)(IMes)Cl, 3ia, 7ca’ and 7da’ reported in this paper have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2192163, 2192164, 2192165, 2192166 and 2192167 (Supplementary Data 2). Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of the study, including experimental procedures and compound characterization, are available within the paper and its Supplementary Information, or from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Longlong Xi, Minyan Wang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-39753-2.
References
- 1.Bergman RG. C–H activation. Nature. 2007;446:391–393. doi: 10.1038/446391a. [DOI] [PubMed] [Google Scholar]
- 2.Wencel-Delord J, Dröge T, Liu F, Glorius F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 2011;40:4740–4761. doi: 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]
- 3.Dong Z, et al. Transition-metal-catalyzed C–H alkylation using alkenes. Chem. Rev. 2017;117:9333–9403. doi: 10.1021/acs.chemrev.6b00574. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Lan J, You J. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem. Rev. 2017;117:8787–8863. doi: 10.1021/acs.chemrev.6b00567. [DOI] [PubMed] [Google Scholar]
- 5.Wang C-S, Dixneuf PH, Soulé J-F. Photoredox catalysis for building C–C bonds from C(sp2)–H bonds. Chem. Rev. 2018;118:7532–7585. doi: 10.1021/acs.chemrev.8b00077. [DOI] [PubMed] [Google Scholar]
- 6.Davies HML, Liao K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 2019;3:347–360. doi: 10.1038/s41570-019-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandeepan P, et al. 3d Transition metals for C–H activation. Chem. Rev. 2019;119:2192–2452. doi: 10.1021/acs.chemrev.8b00507. [DOI] [PubMed] [Google Scholar]
- 8.Guillemard L, Kaplaneris N, Ackermann L, Johansson MJ. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 2021;5:522–545. doi: 10.1038/s41570-021-00300-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Prabagar B, Shi Z. Modern strategies for C‒H functionalization of heteroarenes with alternative coupling partners. Chemistry. 2021;7:2585–2634. doi: 10.1016/j.chempr.2021.08.001. [DOI] [Google Scholar]
- 10.Dalton T, Faber T, Glorius F. C–H Activation: Toward sustainability and applications. ACS Cent. Sci. 2021;7:245–261. doi: 10.1021/acscentsci.0c01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giri R, et al. Transition metal-catalyzed C–H activation reactions: diastereoselectivity and enantioselectivity. Chem. Soc. Rev. 2009;38:3242–3272. doi: 10.1039/b816707a. [DOI] [PubMed] [Google Scholar]
- 12.Wright JS, Scott PJH, Steel PG. Iridium-catalysed C–H borylation of heteroarenes: balancing steric and electronic regiocontrol. Angew. Chem. Int. Ed. 2021;60:2796–2821. doi: 10.1002/anie.202001520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C−H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colby AA, Bergman RG, Ellman JA. Rhodium-catalyzed C−C bond formation via heteroatom-directed C−H bond activation. Chem. Rev. 2010;110:624–655. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang F, Spring DR. Arene C–H functionalisation using a removable/modifiable or a traceless directing group strategy. Chem. Soc. Rev. 2014;43:6906–6919. doi: 10.1039/C4CS00137K. [DOI] [PubMed] [Google Scholar]
- 16.Sambiagio C, et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018;47:6603–6743. doi: 10.1039/C8CS00201K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, et al. Transition-metal-catalyzed, coordination-assisted functionalization of nonactivated C(sp3)–H bonds. Chem. Rev. 2021;121:14957–15074. doi: 10.1021/acs.chemrev.1c00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leow D, Li G, Mei T-S, Yu J-Q. Activation of remote meta-C−H bond assisted by an end-on template. Nature. 2012;486:518–522. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang R, Li G, Yu J-Q. Conformation-induced remote meta-C−H activation of amines. Nature. 2014;507:215–220. doi: 10.1038/nature12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H-J, et al. Rh(III)-catalyzed meta-C–H alkenylation with alkynes. J. Am. Chem. Soc. 2019;141:76–79. doi: 10.1021/jacs.8b11038. [DOI] [PubMed] [Google Scholar]
- 21.Dutta U, Maiti S, Bhattacharya T, Maiti D. Arene diversification through distal C(sp2)−H functionalization. Science. 2021;372:eabd5992. doi: 10.1126/science.abd5992. [DOI] [PubMed] [Google Scholar]
- 22.Dutta U, Maiti D. Emergence of pyrimidine-based meta-directing group: Journey from weak to strong coordination in diversifying meta-C–H functionalization. Acc. Chem. Res. 2022;55:354–372. doi: 10.1021/acs.accounts.1c00629. [DOI] [PubMed] [Google Scholar]
- 23.He C, Whitehurst WG, Gaunt MJ. Palladium-catalyzed C(sp3)–H bond functionalization of aliphatic amines. Chem. 2019;5:1031–1058. doi: 10.1016/j.chempr.2018.12.017. [DOI] [Google Scholar]
- 24.Trowbridge A, Walton SM, Gaunt MJ. New strategies for the transition-metal catalyzed synthesis of aliphatic amines. Chem. Rev. 2020;120:2613–2692. doi: 10.1021/acs.chemrev.9b00462. [DOI] [PubMed] [Google Scholar]
- 25.Herzon SB, Hartwig JF. Direct, Catalytic hydroaminoalkylation of unactivated olefins with N-alkyl arylamines. J. Am. Chem. Soc. 2007;129:6690–6691. doi: 10.1021/ja0718366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koperniku A, Foth PJ, Sammis GM, Schafer LL. Zirconium hydroaminoalkylation. An alternative disconnection for the catalytic synthesis of α-arylated primary amines. J. Am. Chem. Soc. 2019;141:18944–18948. doi: 10.1021/jacs.9b10465. [DOI] [PubMed] [Google Scholar]
- 27.Manßen M, Schafer LL. Titanium catalysis for the synthesis of fine chemicals—development and trends. Chem. Soc. Rev. 2020;49:6947–6994. doi: 10.1039/D0CS00229A. [DOI] [PubMed] [Google Scholar]
- 28.DiPucchio RC, et al. Direct, catalytic α-alkylation of N-heterocycles by hydroaminoalkylation: Substrate effects for regiodivergent product formation. J. Am. Chem. Soc. 2021;143:11243–11250. doi: 10.1021/jacs.1c05498. [DOI] [PubMed] [Google Scholar]
- 29.Manßen M, Schafer LL. Early transition metal-catalyzed hydroaminoalkylation. Trends Chem. 2021;3:428–429. doi: 10.1016/j.trechm.2020.11.007. [DOI] [Google Scholar]
- 30.DiPucchio RC, Rosca S-C, Schafer LL. Hydroaminoalkylation for the catalytic addition of amines to alkenes or alkynes: Diverse mechanisms enable diverse substrate scope. J. Am. Chem. Soc. 2022;144:11459–11481. doi: 10.1021/jacs.1c10397. [DOI] [PubMed] [Google Scholar]
- 31.McNally A, Haffemayer B, Collins BSL, Gaunt MJ. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature. 2014;510:129–133. doi: 10.1038/nature13389. [DOI] [PubMed] [Google Scholar]
- 32.He C, Gaunt MJ. Ligand-enabled catalytic C−H arylation of aliphatic amines by a four-membered ring cyclopalladation pathway. Angew. Chem. Int. Ed. 2015;54:15840–15844. doi: 10.1002/anie.201508912. [DOI] [PubMed] [Google Scholar]
- 33.Willcox D, et al. A general catalytic β-C–H carbonylation of aliphatic amines to β-lactams. Science. 2016;354:851–857. doi: 10.1126/science.aaf9621. [DOI] [PubMed] [Google Scholar]
- 34.Stephens DE, Lariono OV. Recent advances in the C–H-functionalization of the distal positions in pyridines and quinolones. Tetrahedron. 2015;71:8683–8716. doi: 10.1016/j.tet.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabagar B, Yang Y, Shi Z. Site-Selective C−H functionalization to access the arene backbone of indoles and quinolines. Chem. Soc. Rev. 2021;50:11249–11269. doi: 10.1039/D0CS00334D. [DOI] [PubMed] [Google Scholar]
- 36.Lewis JC, Bergman RG, Ellman JA. Rh(I)-Catalyzed alkylation of quinolines and pyridines via C−H bond activation. J. Am. Chem. Soc. 2007;129:5332–5333. doi: 10.1021/ja070388z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berman AM, Lewis JC, Bergman RG, Ellman JA. Rh(I)-catalyzed direct arylation of pyridines and quinolines. J. Am. Chem. Soc. 2008;130:14926–14927. doi: 10.1021/ja8059396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman AM, Bergman RG, Ellman JA. Rh(I)-catalyzed direct arylation of azines. J. Org. Chem. 2010;75:7863–7868. doi: 10.1021/jo101793r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye M, Gao G-L, Yu J-Q. Ligand-promoted C-3 selective C–H olefination of pyridines with Pd catalysts. J. Am. Chem. Soc. 2011;133:6964–6967. doi: 10.1021/ja2021075. [DOI] [PubMed] [Google Scholar]
- 40.Nakao Y, Yamada Y, Kashihara N, Hiyama T. Selective C-4 alkylation of pyridine by nickel/Lewis acid catalysis. J. Am. Chem. Soc. 2010;132:13666–13668. doi: 10.1021/ja106514b. [DOI] [PubMed] [Google Scholar]
- 41.Tsai C-C, et al. Bimetallic nickel aluminun mediated para-selective alkenylation of pyridine: direct observation of η2,η1-pyridine Ni(0)-Al(iii) intermediates prior to C–H bond activation. J. Am. Chem. Soc. 2010;132:11887–11889. doi: 10.1021/ja1061246. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, et al. A radical approach for the selective C–H borylation of azines. Nature. 2021;595:677–683. doi: 10.1038/s41586-021-03637-6. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, et al. Phosphorus-mediated sp2–sp3 couplings for C–H fluoroalkylation of azines. Nature. 2021;594:217–222. doi: 10.1038/s41586-021-03567-3. [DOI] [PubMed] [Google Scholar]
- 44.Hwang H, Kim J, Jeong J, Chang S. Regioselective introduction of heteroatoms at the C-8 position of quinoline N-oxides: Remote C–H activation using N-oxide as a stepping stone. J. Am. Chem. Soc. 2014;136:10770–10776. doi: 10.1021/ja5053768. [DOI] [PubMed] [Google Scholar]
- 45.Shukla RK, Nair AM, Khan S, Volla CMR. Cobalt-catalyzed C8-dienylationof quinoline-N-oxides. Angew. Chem. Int. Ed. 2020;59:17042–17048. doi: 10.1002/anie.202003216. [DOI] [PubMed] [Google Scholar]
- 46.Stephens DE, et al. Palladium-catalyzed C8-selective C−H arylation of quinoline N‑oxides: insights into the electronic, steric, and solvation effects on the site selectivity by mechanistic and DFT computational studies. ACS Catal. 2015;5:167–175. doi: 10.1021/cs501813v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapley, J. R., Samkoff, D. E., Bueno, C. & Churchill, M. R. Reaction of Os3(CO)10(NCCH3)2 with imidazole and related aromatic heterocycles. The crystal and molecular structure of (.mu.-H)Os3(CO)10 (.mu.-3,4-.eta.2-N2C3H3. Inorg. Chem. 21, 634–639 (1982).
- 48.Fukuyama T, et al. Ru3(CO)12-Catalyzed site-selective carbonylation reactions at a C−H Bond in aza-heterocycles. J. Am. Chem. Soc. 1998;120:11522–11523. doi: 10.1021/ja982794b. [DOI] [Google Scholar]
- 49.Kwak J, Kim M, Chang S. Rh(NHC)-catalyzed direct and selective arylation of quinolines at the 8-position. J. Am. Chem. Soc. 2011;133:3780–3783. doi: 10.1021/ja111670s. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Tanaka K, Yu J-Q. Remote site-selective C–H activation directed by a catalytic bifunctional template. Nature. 2017;543:538–542. doi: 10.1038/nature21418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramakrishna K, et al. Coordination assisted distal C–H alkylation of fused heterocycles. Angew. Chem. Int. Ed. 2019;58:13808–13812. doi: 10.1002/anie.201907544. [DOI] [PubMed] [Google Scholar]
- 52.Shi H, et al. Differentiation and functionalization of remote C–H bonds in adjacent positions. Nat. Chem. 2020;12:399–404. doi: 10.1038/s41557-020-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan Z, et al. Molecular editing of aza-arene C–H bonds by distance, geometry and chirality. Nature. 2022;610:87–93. doi: 10.1038/s41586-022-05175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Q, Meng G, Nolan SP, Szostak M. N-Heterocyclic carbene complexes in C–H activation reactions. Chem. Rev. 2020;120:1981–2048. doi: 10.1021/acs.chemrev.9b00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ano Y, Tobisu M, Chatani N. Palladium-catalyzed direct ethynylation of C(sp3)–H bonds in aliphatic carboxylic acid derivatives. J. Am. Chem. Soc. 2011;133:12984–12986. doi: 10.1021/ja206002m. [DOI] [PubMed] [Google Scholar]
- 56.He J, Wasa M, Chan KSL, Yu J-Q. Palladium(0)-catalyzed alkynylation of C(sp3)−H bonds. J. Am. Chem. Soc. 2013;135:3387–3390. doi: 10.1021/ja400648w. [DOI] [PubMed] [Google Scholar]
- 57.Viart HM-F, Bachmann A, Kayitare W, Sarpong R. β‑Carboline amides as intrinsic directing groups for C(sp2)−H functionalization. J. Am. Chem. Soc. 2017;139:1325–1329. doi: 10.1021/jacs.6b12569. [DOI] [PubMed] [Google Scholar]
- 58.Liao G, et al. Scalable, Stereocontrolled formal syntheses of (+)-Isoschizandrin and (+)-Steganone: Development and applications of palladium(II)-catalyzed atroposelective C−H alkynylation. Angew. Chem. Int. Ed. 2018;57:3661–3665. doi: 10.1002/anie.201713106. [DOI] [PubMed] [Google Scholar]
- 59.Mondal A, et al. Sterically controlled late-stage C−H alkynylation of arenes. J. Am. Chem. Soc. 2019;141:18662–18667. doi: 10.1021/jacs.9b10868. [DOI] [PubMed] [Google Scholar]
- 60.Neue B, Araneda JF, Piers WE, Parvez M. BN-Dibenzo[a,o]picenes: analogues of an unknown polycyclic aromatic hydrocarbon. Angew. Chem. Int. Ed. 2013;52:9966–9969. doi: 10.1002/anie.201302911. [DOI] [PubMed] [Google Scholar]
- 61.Qi X, Li Y, Bai R, Lan Y. Mechanism of rhodium-catalyzed C−H functionalization: advances in theoretical investigation. Acc. Chem. Res. 2017;50:2799–2808. doi: 10.1021/acs.accounts.7b00400. [DOI] [PubMed] [Google Scholar]
- 62.Davies DL, Macgregor SA, McMullin CL. Computational studies of carboxylate-assisted C−H activation and functionalization at group 8−10 transition metal centers. Chem. Rev. 2017;117:8649–8709. doi: 10.1021/acs.chemrev.6b00839. [DOI] [PubMed] [Google Scholar]
- 63.Lapointe D, Fagnou K. Overview of the mechanistic work on the concerted metallation-deprotonation pathway. Chem. Lett. 2010;39:1118–1126. doi: 10.1246/cl.2010.1118. [DOI] [Google Scholar]
- 64.Funes-Ardoiz I, Maseras F. Oxidative coupling mechanisms: current state of understanding. ACS Catal. 2018;8:1161–1172. doi: 10.1021/acscatal.7b02974. [DOI] [Google Scholar]
- 65.Balcells D, Clot E, Eisenstein O. C−H bond activation in transition metal species from a computational perspective. Chem. Rev. 2010;110:749–823. doi: 10.1021/cr900315k. [DOI] [PubMed] [Google Scholar]
- 66.Jiang Y-Y, Man X, Bi S. Advances in theoretical study on transition-metal-catalyzed C−H activation. Sci. China: Chem. 2016;59:1448–1466. doi: 10.1007/s11426-016-0330-3. [DOI] [Google Scholar]
- 67.Falivene L, et al. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 2019;11:872–879. doi: 10.1038/s41557-019-0319-5. [DOI] [PubMed] [Google Scholar]
- 68.Falivene L, et al. SambVca 2. A web tool for analyzing catalytic pockets with topographic steric maps. Organometallics. 2016;35:2286–2293. doi: 10.1021/acs.organomet.6b00371. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Crystallographic data for the structures of Rh(cod)(dtbpy)Cl, Rh(cod)(IMes)Cl, 3ia, 7ca’ and 7da’ reported in this paper have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2192163, 2192164, 2192165, 2192166 and 2192167 (Supplementary Data 2). Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of the study, including experimental procedures and compound characterization, are available within the paper and its Supplementary Information, or from the corresponding author upon reasonable request.