Table 2.
Names and chemical structures of some DPP-4 inhibitors currently on market.
| Number | Name | Manufacturer | Trade name | Chemical structure | Date of approval by FDA | Ref. |
|---|---|---|---|---|---|---|
| 1 | Sitagliptin | Merck | Januvia™; Glactiv®; Tesavel® | 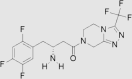 |
16th Oct, 2006 | 62, 63, 64, 65 |
| 2 | Saxagliptin | AstraZeneca | Onglyza™ | 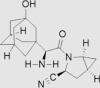 |
31st Jul, 2009 | 66, 67 |
| 3 | Linagliptin | Eli Lilly and Company | Trajenta® | 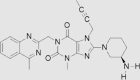 |
2nd May, 2011 | 68, 69, 70, 71 |
| 4 | Alogliptin | GlaxoSmithKline | Tanzeum |  |
25th Jan, 2013 | 72, 73, 74, 75 |
| 5 | Vildagliptin | Novartis | Galvus®; Jalra®; Xiliarx® | 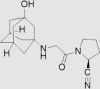 |
Not by FDA (30th Nov, 2008 by European Medicines Agency) | 76, 77, 78, 79 |
| 6 | Gemigliptin | LG Life Sciences | Zemiglo® | 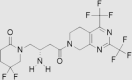 |
Not by FDA (Jun, 2012 by Korea Food and Drug Administration) | 80, 81, 82, 83, 84 |
| 7 | Teneligliptin | Mitsubishi Tanabe Pharma | Tenelia® | 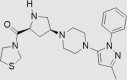 |
Not by FDA (Sep, 2012 by Pharmaceuticals and Medical Devices Agency) | 85, 86, 87, 88 |
| 8 | Omarigliptin | Merck | Marizev® | 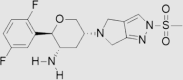 |
Not by FDA (Nov, 2015 by Pharmaceuticals and Medical Devices Agency) | 89, 90 |
