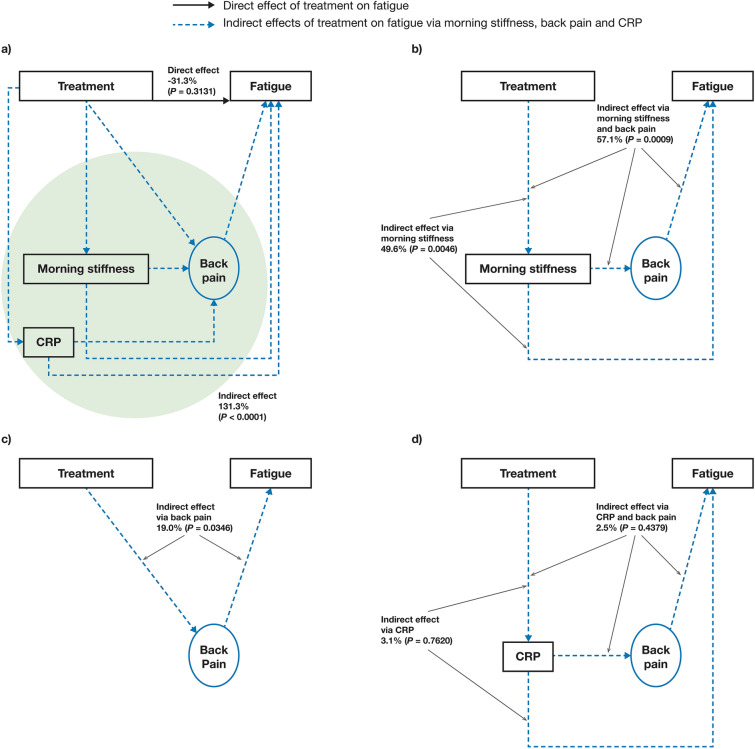
Fig. 2.
Effects of tofacitinib treatment on fatigue in initial mediation model A (FACIT-F-based). Estimated: a direct effect and overall indirect effect,a b indirect effect via morning stiffness and back pain or morning stiffness alone, c indirect effect via back pain alone, and d indirect effect via C-reactive protein and back pain or C-reactive protein alone. Results based on pooled data from phase 2 (NCT01786668) and phase 3 (NCT03502616) studies. Treatment was the independent binary variable (tofacitinib 5 mg BID versus placebo). Fatigue was based on FACIT-F. Back pain was represented by total back pain due to AS on average during last week and nocturnal spinal pain due to AS on average during last week. Morning stiffness was considered an observed variable and was represented by the mean of BASDAI Q5 and Q6. CRP was considered an observed variable. aGreen shading denotes all indirect effects of tofacitinib on fatigue via back pain, morning stiffness and CRP. AS ankylosing spondylitis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BID twice daily, CRP C-reactive protein, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, Q question