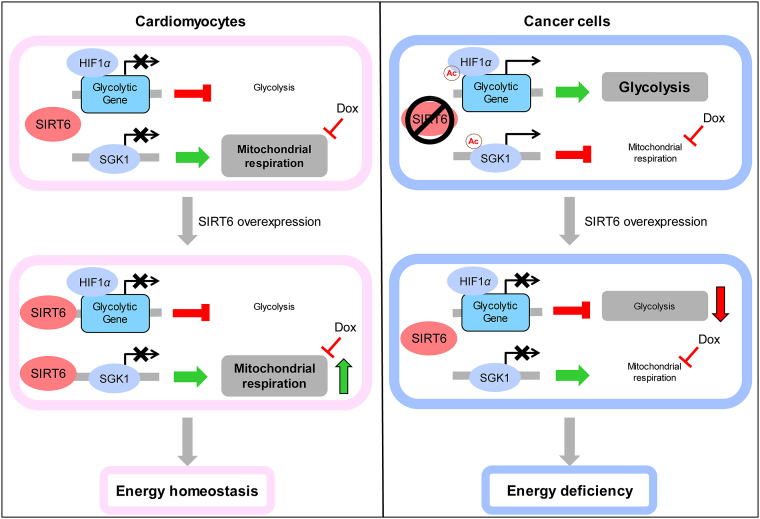
Figure 10.
Schematic framework of the SIRT6-mediated differential responses to Dox between cardiomyocytes and cancer cells. Dox-impaired mitochondrial DNA led to metabolic remodeling of decreased mitochondrial respiration and increased glycolysis, thus inhibiting cellular ATP production, which were responsible for Dox-induced cytotoxicity. SIRT6 has been reported to function as a histone H3K9 deacetylase and inhibits the transcription of multiple glycolytic genes. Meanwhile, SIRT6 regulated mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis by deacetylating and inhibiting SGK1, thus improving mitochondrial respiration when mitochondrial function is impaired by Dox. Thus, the differences in metabolic patterns between cardiomyocytes and cancer cells are responsible for the differential regulation of SIRT6 on energy production. In cardiomyocytes that depend on mitochondrial respiration for energy production, SIRT6 overexpression reversed the Dox-induced reduction in ATP production and improved cellular energy supply by increasing mitochondrial respiration. In cancer cells that depend on glycolysis for energy production, SIRT6 overexpression enhanced the Dox-induced reduction in ATP production and aggravated cellular energy deficiency by inhibiting glycolysis. Therefore, SIRT6 overexpression effectively reversed Dox-induced metabolic remodeling by increasing mitochondrial respiration and inhibiting glycolysis, which differentially regulated ATP production in cardiomyocytes and cancer cells.