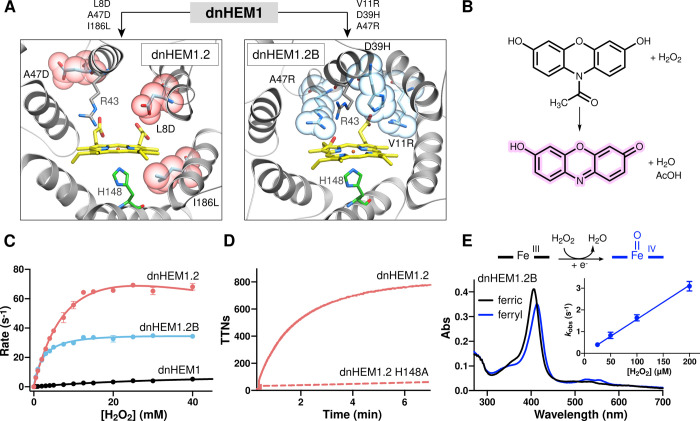
Figure 4.
Directed evolution converts dnHEM1 into a proficient peroxidase with a stable high valent ferryl intermediate. (A) AlphaFold252 predicted models of dnHEM1.2 and dnHEM1.2B indicating the mutations accumulated as a result of divergent directed evolution. Sites of mutation for dnHEM1.2 and dnHEM1.2B are shown as atom colored ball and sticks with purple or blue carbons, respectively. The heme cofactor is shown as gray atom colored ball and sticks and CPK spheres. (B) Chemical scheme showing the conversion of Amplex Red dye to resorufin mediated by hydrogen peroxide and dnHEM1 variants. (C) Michaelis–Menten plots under saturating concentrations of Amplex Red for dnHEM1 (black, kcat = 9.5 ± 0.2 s–1, KM[H2O2] = 36.7 ± 1.6 mM), dnHEM1.2 (red, kcat = 129.5 ± 8.7 s–1, KM[H2O2] = 11.5 ± 1.2 mM, KI = 58.9 ± 10.7 mM) and dnHEM1.2B (blue, kcat = 37.0 ± 0.3 s–1, KM[H2O2] = 2.0 ± 0.1 mM). Error bars represent SD n = 3. (D) Time course of resorufin formation by dnHEM1.2 (red solid line, TTNs = 788 ± 18) and dnHEM1.2 H148A (red dotted line). (E) Formation of a ferryl intermediate (blue) in dnHEM1.2B upon oxidation of the ferric enzyme (black line) with H2O2. The observed neutral ferryl heme state is most likely formed via rapid single electron transfer to a transient porphyrin-π cation radical species, with a redox active amino acid side chain being the most likely electron donor. Inset: A linear fit of kobs vs [H2O2] was used to derive a bimolecular rate constant of (1.6 ± 0.05) × 104 M–1 s–1 for ferryl heme formation. Error bars represent SD n = 3.