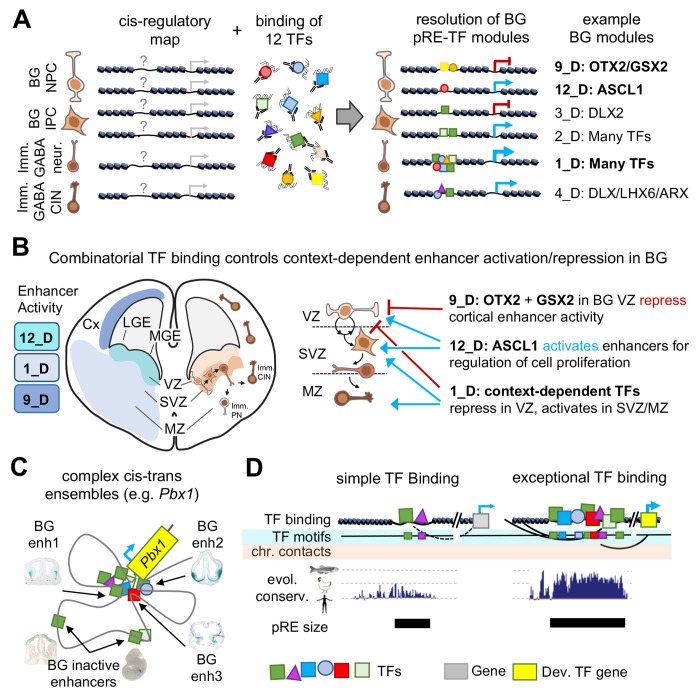
Figure 7 – Cis-trans interactions underlying gene regulation driving GABAergic neurogenesis.
(A) Chromatin accessibility maps identify pREs, but TF binding is necessary to understand mechanisms and functional relevance of pRE activity. TF binding can direct either activation or repression of enhancer activity. Here we identify pRE-TF modules that drive specific regulatory activity in developing mouse BG, with representative examples depicted. Bold BG modules in (A) are highlighted in (B). (B) Combinatorial TF binding defines context-dependent patterns of enhancer activation and repression in embryonic BG. Three example cis-trans modules identified here are shown, with the enhancer activity and schematic of activity across VZ, SVZ, and MZ. (C) Developmental TF genes (i.e. Pbx1) relevant to embryonic BG have complex cis-regulatory landscapes and generally include multiple cis-trans regulatory modules. (D) Comparison of enhancers with simple versus complex TF binding identified in embryonic BG. Enhancers with exceptional TF binding also feature high density of TF binding motifs, complex chromosomal contacts, strong evolutionary conservation across the vertebrate tree (human, chicken, zebrafish conservation represented), and increased base pair size. Abbreviations: Imm. CIN: immature cortical interneurons, Imm. PN: immature projection neurons, BG NPC: basal ganglia neural progenitor cell, BG IPC: basal ganglia intermediate progenitor cell.