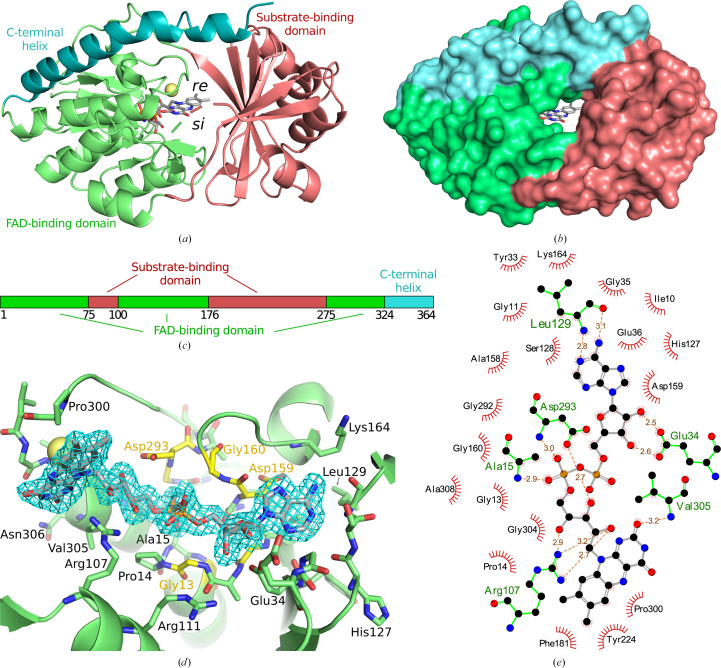
Figure 3.
Structural analysis of SmTetX. The substrate-binding domain is coloured red, the FAD-binding domain green and the C-terminal helix blue. C atoms of FAD are shown in grey in stick representation and a chloride anion is shown as a yellow sphere. (a, b) Overall fold represented in secondary structure (a) and surface (b) views along the access tunnel to the re site. (c) Schematic diagram of the SmTetX domain structure; the numbers correspond to the native amino-acid sequence. (d) The binding of FAD in combined stick and secondary-structure representation: a view from the flavin si site. The 2mF o − DF c electron density is contoured in blue at the 1σ level. C atoms of the residues belonging to the GXGXXG, DG and GD binding motifs are coloured yellow. (e) Interactions of FAD with the protein. Hydrogen bonds are shown in orange with distances in Å. The graphics were prepared in PyMOL 2.5 (Schrödinger) and LigPlot+ (Laskowski & Swindells, 2011 ▸).