-
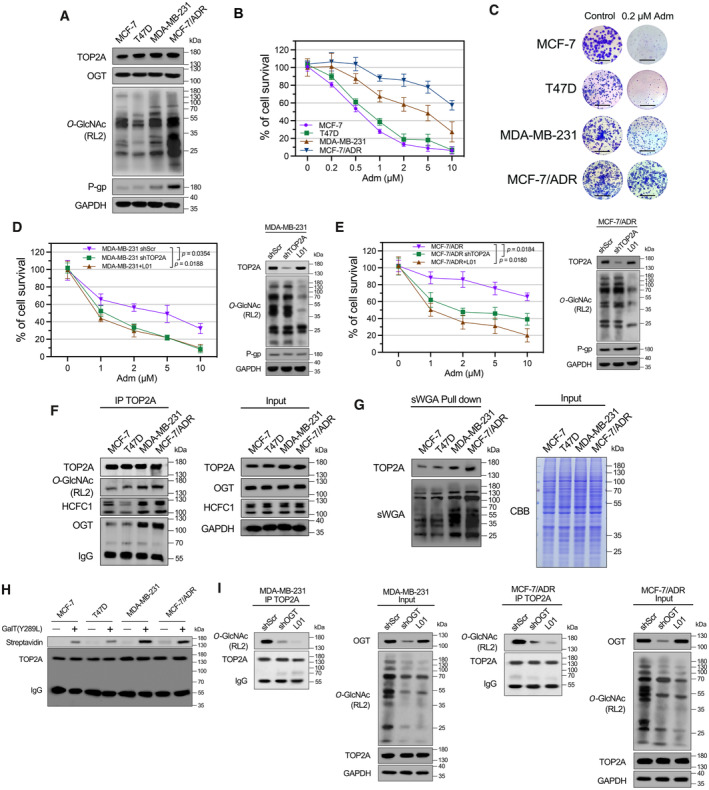
A
Expression of TOP2A, OGT, P‐gp, and cellular O‐GlcNAcylation in Adm‐sensitive and Adm‐resistant breast cancer cell lines was analyzed by Western blot analysis.
-
B
Breast cancer cells were treated with increasing doses of Adm alone for 48 h, and the cell viability was assessed using CCK‐8 assay. n = 3 biological replicates. The data are presented as means ± SD.
-
C
Colony formation assays were performed in breast cancer cells exposed to 0.2 μM Adm. The micrograph scale bar represents 5 mm.
-
D, E
MDA‐MB‐231 or MCF‐7/ADR cells were transfected with TOP2A shRNA (shTOP2A) or treated with 50 μM L01 and then incubated with the indicated doses of Adm for 48 h. Cell viability was assessed with a CCK‐8 assay. Protein expression was analyzed by Western blot. shScr, scrambled shRNA. n = 3 biological replicates. Paired t‐test was used for statistical comparison. P‐value was indicted. The data are presented as means ± SD.
-
F
TOP2A co‐IP was performed, and the immunoprecipitated fractions were analyzed by Western blot for the indicated proteins.
-
G
sWGA lectin pull‐down was performed, and precipitated fractions were analyzed by Western blot and lectin blot. CBB, Coomassie Brilliant Blue staining.
-
H
The O‐GlcNAc enzymatic labeling system was employed to confirm the O‐GlcNAcylation of endogenous TOP2A in breast cancer cells. TOP2A IP was performed, and immunoprecipitated fractions were incubated with Y289L GalT1. Streptavidin beads were used to capture O‐GlcNAc proteins. An anti‐TOP2A antibody was used for immunoblotting.
-
I
MDA‐MB‐231 or MCF‐7/ADR cells were transfected with OGT shRNA (shOGT) or treated with 50 μM L01 for 48 h. TOP2A co‐IP was performed, and the immunoprecipitated fractions were analyzed by Western blot for the indicated proteins.