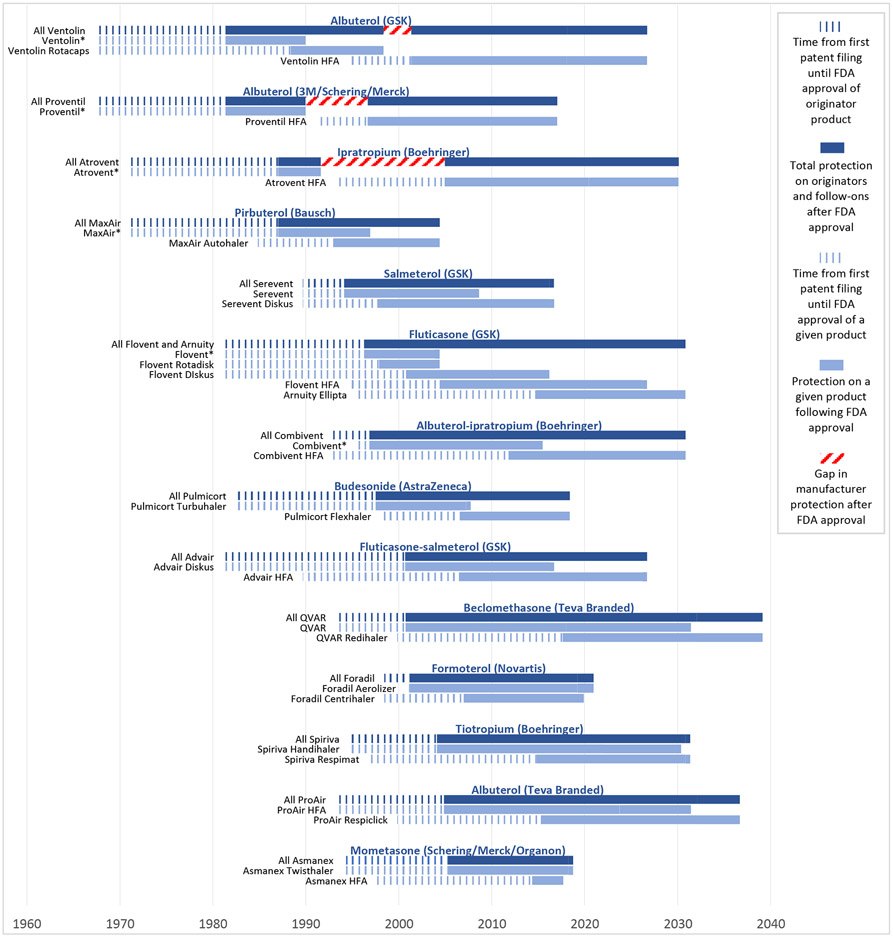
Exhibit 4: Device hops by inhaler manufacturers.
Source: FDA Orange Book, Drugs@FDA, authors’ analysis
GSK: GlaxoSmithKline
*Denotes an inhaler containing chlorofluorocarbons (CFCs), which were removed from the market by the Food and Drug Administration beginning in the 2000s.
This figure shows how manufacturers have preserved monopolies on inhaled medications by pairing old active ingredients with new devices. The notched dark blue bars represent the time that elapsed between the first patents filed for an originator product and FDA approval of that product. The solid dark blue bars represent the time that elapsed between FDA approval of an originator product and the last-to-expire exclusivity or patent on the originator or follow-on products. This reflects the total protection that a manufacturer has obtained on inhalers with a given active ingredient (or ingredients). The notched grey bars represent the time that elapsed between the first patents filings for a given product and FDA approval of that product. The solid grey bars represent the time that elapsed between FDA approval of a given product and the last-to-expire exclusivity or patent on that product. A median of 40.3 years (IQR 33.9-45.8) elapsed between the first patent filed on originator inhalers and the last-to-expire exclusivity or patent on these inhalers or their follow-ons. Manufacturers enjoyed a median of 28.1 years (IQR 21.3-33.5) of protection on these inhalers after FDA approval of the originator.