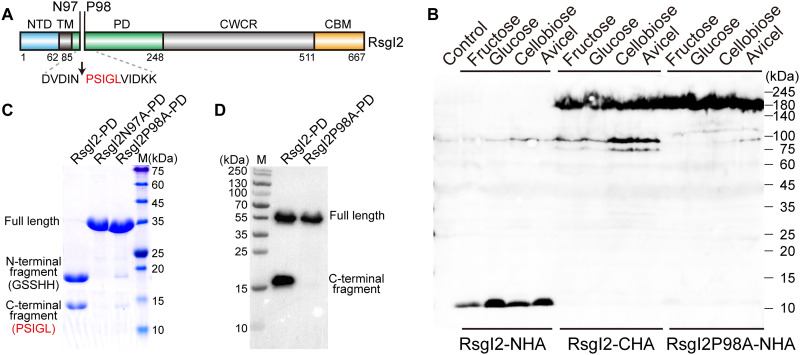
Fig. 1. Identification of the autocleavage phenomenon and the cleavage site of C. thermocellum RsgI2.
(A) Schematic representation of the composition and the location of the autocleavage site of RsgI2. RsgI2 contains an NTD, a transmembrane helix (TM), a PD, a probable cell wall–crossing region (CWCR) and a C-terminal substrate-binding CBM3b domain (CBM). The amino acid sequence around the autocleavage site is shown, and the first five amino acid residues identified by N-terminal protein sequencing are colored in red. (B) Western-blot analysis of RsgI2 expressed in C. thermocellum. The mutant ΔrsgI2 (control), the ΔrsgI2 bearing the plasmids to express RsgI2 with either an N- or C-terminal HA-tag (RsgI2-NHA and RsgI2-CHA), and the P98A mutant RsgI2P98A-NHA were cultured on the different designated carbon sources, and the resultant labeled RsgI2 components were detected by an anti–HA-tag antibody. (C) SDS–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the purified SMT3-RsgI2-PD protein and its N97A and P98A mutants. The results of N-terminal protein sequencing for the two bands of SMT3-RsgI2-PD are denoted parenthetically on the left. (D) RsgI2-PD fused with an N-terminal glutathione S-transferase (GST) and a C-terminal FLAG tag (RsgI2-PD) and its P98A mutant (RsgI2P98A-PD) were expressed using the PURE cell-free transcription/translation system and analyzed by Western blotting using an anti-FLAG antibody.