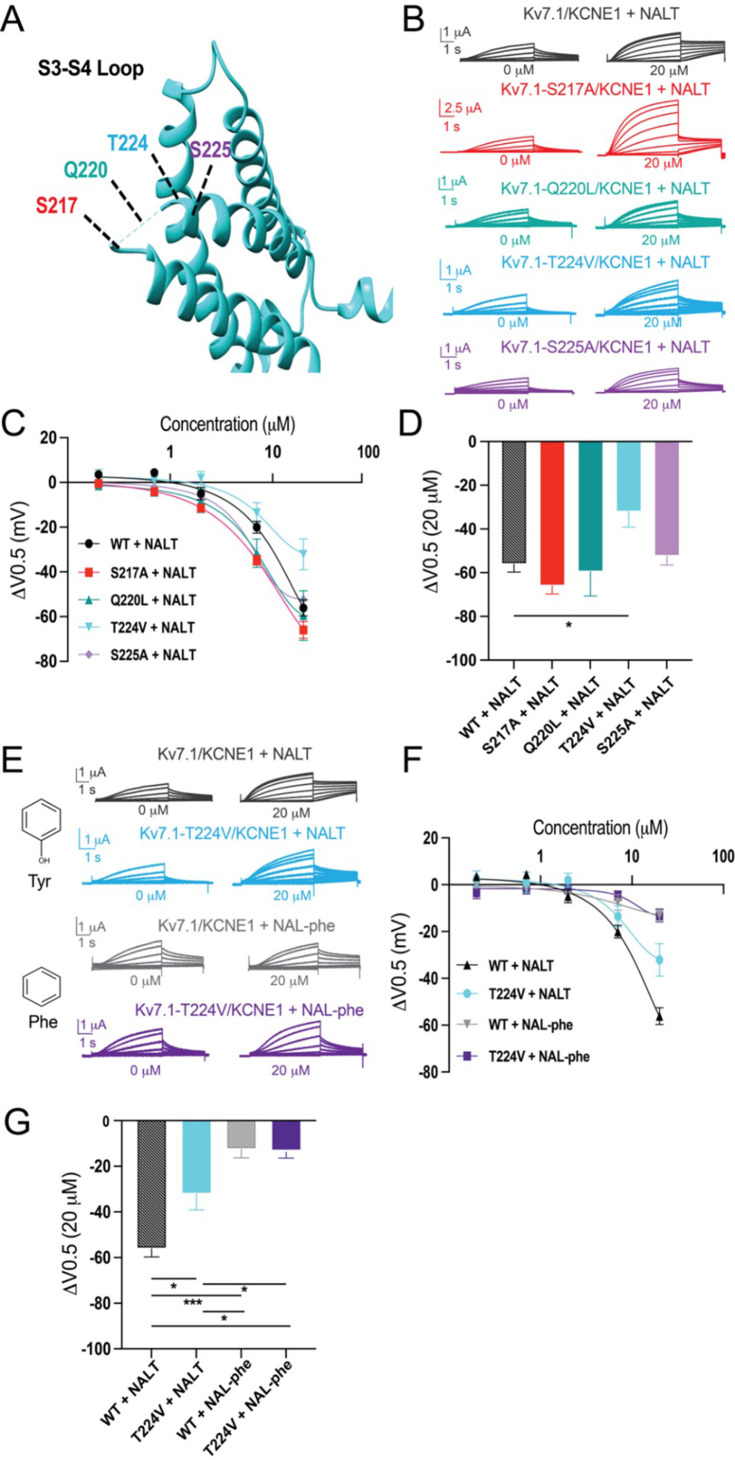
Figure 6. Loop is the locus for hydrogen bonding interactions with tyrosine polyunsaturated fatty acids (PUFAs).
(A) Top view of KV7.1 voltage-sensing domain (VSD) highlighting mutated residues in the S3–S4 loop. (B) Representative traces of WT KV7.1/KCNE1 (black), KV7.1-S217A/KCNE1 (red), KV7.1-Q220L/KCNE1 (teal), KV7.1-T224V/KCNE1 (cyan), and KV7.1-S225A/KCNE1 (purple) with 0 μM (left) and 20 μM (right) NALT. (C) ΔV0.5 dose–response curve for WT KV7.1/KCNE1 (n = 4), KV7.1-S217A/KCNE1 (n = 5), KV7.1-Q220L/KCNE1 (n = 3), KV7.1-T224V/KCNE1 (n = 4), and KV7.1-S225A/KCNE1 (n = 7) with NALT. (D) Maximum effects on ΔV0.5 (at 20 μM) for WT and S3–S4 loop mutations. Asterisks indicate statistically significant differences determined by one-way ANOVA. (E) Representative traces of WT KV7.1/KCNE1 with NALT (black) and NAL-phe (gray) compared to KV7.1-T224V/KCNE1 with NALT (cyan) and NAL-phe (dark purple), KV7.1-S217A/KCNE1 (red), KV7.1-Q220L/KCNE1 (teal), KV7.1-T224V/KCNE1 (cyan), and Kv7.1-S225A/KCNE1 (purple) with 0 μM (left) and 20 μM (right) NALT. (F) ΔV0.5 dose–response curve for WT KV7.1/KCNE1 and KV7.1-T224V/KCNE1 with NALT and NAL-phe. (G) Maximum effects on ΔV0.5 (at 20 μM) for WT KV7.1/KCNE1 (n = 4) and KV7.1-T224V/KCNE1 with NALT (n = 4) and NAL-phe (n = 7). Asterisks indicate statistically significant differences determined by one-way ANOVA. Values for all compounds and concentrations available in Figure 6—source data 1.