Abstract
The non-selective opioid receptor antagonist, naltrexone is one of the most prescribed medications for treating alcohol and opioid addiction. Despite decades of clinical use, the mechanism(s) by which naltrexone reduces addictive behavior remains unclear. Pharmaco-fMRI studies to date have largely focused on naltrexone’s impact on brain and behavioral responses to drug or alcohol cues or on decision-making circuitry. We hypothesized that naltrexone’s effects on reward-associated brain regions would associate with reduced attentional bias (AB) to non-drug, reward-conditioned cues. Twenty-three adult males, including heavy and light drinkers, completed a two-session, placebo-controlled, double-blind study testing the effects of acute naltrexone (50 mg) on AB to reward-conditioned cues and neural correlates of such bias measured via fMRI during a reward-driven AB task. While we detected significant AB to reward-conditioned cues, naltrexone did not reduce this bias in all participants. A whole-brain analysis found that naltrexone significantly altered activity in regions associated with visuomotor control regardless of whether a reward-conditioned distractor was present. A region-of-interest analysis of reward-associated areas found that acute naltrexone increased BOLD signal in the striatum and pallidum. Moreover, naltrexone effects in the pallidum and putamen predicted individual reduction in AB to reward-conditioned distractors. These findings suggest that naltrexone’s effects on AB primarily reflect not reward processing per se, but rather top-down control of attention. Our results suggest that the therapeutic actions of endogenous opioid blockade may reflect changes in basal ganglia function enabling resistance to distraction by attractive environmental cues, which could explain some variance in naltrexone’s therapeutic efficacy.
Keywords: Alcohol, Attentional bias, Cognitive, Conditioning, fMRI, Naltrexone, Opioids
Introduction
Alcohol use disorder (AUD) is characterized by attentional bias (AB) to alcohol-related stimuli [1], perhaps reflecting a learned response in which alcohol-related cues come to initiate alcohol seeking behaviors. Such AB to substance-related cues is reported to correlate with addiction severity and/or treatment outcomes across multiple substances of abuse [2–7]. Specifically, AB positively correlates with alcohol craving, a measure that may predict relapse risk in individuals with AUD [8]. Moreover, experimentally manipulating AB to alcohol cues can reduce subsequent alcohol craving and consumption [9]. Despite decreased responsiveness to non-drug rewards among individuals with SUDs [10], enhanced AB to non-drug rewards has been associated with alcohol and other substance abuse [11–13], perhaps reflecting a heightened susceptibility to reward conditioning.
Naltrexone, one of few pharmaceuticals approved by the U.S. Food and Drug Administration (FDA) for the treatment of AUD, is a non-selective opioid antagonist that is hypothesized to attenuate alcohol craving and consumption via the endogenous opioid system’s modulation of dopamine release, reducing alcohol’s rewarding effects [14]. Neuroimaging evidence supports this hypothesized mechanism of action, showing that naltrexone reduces alcohol cue-induced responses in the ventral striatum [15,16]. Further, naltrexone has been found to have significant effects on reward-related behaviors, reducing cue-induced responding to non-drug rewarded stimuli [17]. Despite these findings, naltrexone’s efficacy in treating alcohol dependence is highly variable across individuals [18,19], and its specific interaction with and impact upon the reward system remains ill-defined. Better understanding naltrexone’s neurocognitive effects is crucial to improving and personalizing AUD treatment [20].
Several lines of evidence support the idea that naltrexone’s therapeutic benefit may derive in part from reducing AB to alcohol-conditioned cues. First, findings from animal models demonstrate that naltrexone reduces drug-seeking behavior triggered by drug-cue exposure [21–24]. Similarly, naltrexone reduces alcohol cue-induced craving in people with AUD [25,26]. In addition, our prior work suggests that naltrexone reduces attention to salient stimuli that must be ignored [27,28], consistent with other data showing that endogenous opioid blockade affects selective allocation of attention [29,30]. Based on our previous findings, we proposed that naltrexone may reduce AB to reward-conditioned cues due to its putative ability to attenuate cue-induced dopamine release [27,28]. This idea, together with our finding that acute dopamine depletion reduces AB to both alcohol and reward-conditioned cues in heavy drinkers [13], led us to predict that naltrexone would reduce AB to both alcohol and reward-conditioned cues and impact reward-related neural circuitry.
We used a double-blinded, placebo-controlled crossover study design to test the neurocognitive effects of acute naltrexone (50 mg) in light to heavy drinking adult males on three AB tasks. Pairing the non-drug reward AB task with functional MRI, we sought to test the hypothesis that naltrexone’s effects on reward-associated brain regions would reduce AB to non-drug, reward-conditioned cues.
Materials and methods
Participants
Twenty-four healthy males aged 22–39 years (mean=25.7) were recruited for this study from the community surrounding the University of North Carolina at Chapel Hill. Only males were included due to sample size limitations and known sex-dependent effects of naltrexone [31–33]. Participants were excluded for any self-reported neurological or psychiatric disorders (excluding AUD), motor or visual impairments that would affect task performance, or current psychoactive drug use, including medication. To be included in the study, participants were required to be between 22 and 40 years of age, native English speakers, right-handed, and must have completed high-school or received their GED. Additionally, participants were excluded based on any contraindications to MRI or naltrexone. We screened for psychiatric illness using the Mini-International Neuropsychiatric Interview [34]; for individuals enrolled as binge drinkers (see below), current or past AUD was not exclusionary. To ensure a broad distribution of alcohol intake for use as a continuous measure, we recruited participants into two groups that were guided by the National Institute on Alcohol Abuse and Alcoholism’s (NIAAA) definitions of binge drinking (5 drinks/2 hrs) [35]. Participants who reported binge drinking at least once a month over the past year were recruited into the binge drinker group (), while participants with no binges in the past year were recruited into the light/moderate drinker group (). All participants reported lifetime alcohol use and displayed a wide range of recent drinking behaviors (Table 1). Of the 24 participants who consented to participate, one participant in the binge drinking group withdrew from the study due to drug side effects. Two were excluded from neuroimaging analyses due to excessive head motion during MRI scanning and two additional participants were excluded due to incomplete behavioral data from the neuroimaging task. Therefore, the final sample for neuroimaging analyses was 19 participants, and the sample for the fMRI task behavioral analyses was 22 participants, each measured on both placebo and naltrexone. There were two out-of-scanner tasks, and complete data for both placebo and naltrexone visits were available for 22 and 23 subjects on those tasks. Participants gave written, informed consent, as approved by the UNC Office of Human Research Ethics.
Table 1.
Psychometric and substance use questionnaire data.
| (N = 22) | Mean ± SD | Range | Mean ± SD | Range | |
|---|---|---|---|---|---|
| Psychometric | Substance Use | ||||
| BIS Total | 58.3 ± 11.5 | 39–83 | AUDIT Total | 7.2 ± 4.9 | 1–19 |
| Attention | 14.6 ± 3.6 | 10–21 | Consumption | 5.2 ± 2.9 | 1–11 |
| Motor | 23.2 ± 4.6 | 15–33 | Dependence | 0.3 ± 0.66 | 0–2 |
| Non-planning | 20.5 ± 4.8 | 13–30 | Harm | 1.7 ± 2.1 | 0–7 |
| LOC | 9.8 ± 3.6 | 5–21 | Drinks per Week | 7.0 ± 9.2 | 0–40 |
| CAARS | DUSI | 2.7 ± 2.5 | 0–7 | ||
| Inattention | 9.0 ± 4.7 | 0–15 | DMQ-R | ||
| Hyper | 12.3 ± 5.0 | 4–24 | Social | 15.9 ± 5.5 | 5–25 |
| Impulsive | 6.4 ± 3.9 | 1–16 | Coping | 7.9 ± 1.9 | 5–11 |
| ADHD | 8.6 ± 3.0 | 3–13 | Enhancement | 12.8 ± 4.5 | 6–21 |
| DSM-Inattention | 5.6 ± 3.1 | 0–13 | Conformity | 6.6 ± 2.0 | 5–11 |
| DSM-Hyper | 7.2 ± 3.6 | 2–13 | CAUP-Q | ||
| DSM-ADHD | 12.8 ± 5.5 | 2–23 | Binge Score | 17.0 ± 19.4 | 1–86 |
| DASS | U18 Binge Freq. | 0.65 ± 0.88 | 0–3 | ||
| Depression | 2.8 ± 3.6 | 0–15 | 18–21 Binge Freq. | 2.6 ± 2.1 | 0–6 |
| Anxiety | 1.3 ± 1.6 | 0–5 | Past 12 Mo. Binge Freq. | 2.0 ± 2.0 | 0–5 |
| Stress | 5.2 ± 4.7 | 0–16 | Family History Density (%) | 0.12 ± 0.14 | 0–0.4 |
Procedure
This study employed a double-blind, placebo-controlled crossover design, with two separate sessions of ∼5 hrs each; visits were separated by at least five days. Participants were screened for acute alcohol intoxication or recent illicit drug use at the beginning of study session via a breathalyzer test (FC-10, Lifeloc Inc., Wheat Ridge, CO) and a urine drug test (Biotechnostix, Inc., Markham, ON), respectively. After providing informed consent and completing all screening procedures, participants were administered either a naltrexone (50 mg) pill or identical lactose placebo pill. A reward conditioning task and two out-of-scanner alcohol AB tasks commenced three hours after pill ingestion, following published methods [27,28,36], with participants completing a battery of psychometric questionnaires in the interim. Upon completion of behavioral tasks, participants completed the testing phase of the reward task during functional magnetic resonance imaging (fMRI) at the Biomedical Research Imaging Center at UNC, Chapel Hill. Before departure, participants were asked to report any side effects and their best guess as to which pill they had received in the session.
Psychometric and substance use questionnaires
All subjects completed a battery of questionnaires to assess personality, psychiatric symptoms, and substance use measures. These included the Alcohol Use Disorders Identification Test (AUDIT) [37], the Barratt Impulsivity Scale (BIS) [38], the Drug Use Screening inventory (DUSI) – Domain 1 (Substance Use) [39], the Family Tree Questionnaire (FTQ) [40] for a calculation of family history density [41], Rotter’s Locus of Control Scale (LOC) [42], Conners Adult ADHD Rating Scales-Self Report (CAARS-SR) [43], Depression Anxiety Stress Scales (DASS) [44], Drinking Motives Questionnaire-Revised (DMQR) [45], and the Carolina Alcohol Use Pattern Questionnaire [13] to assess current and past binge drinking, which includes questions 10–12 of the Alcohol Use Questionnaire [46], for the calculation of a “binge score” [47].
Behavioral tasks
Reward-driven attentional capture task
We probed AB to monetary reward using a reward-driven attentional bias (RDAB) task, described previously [11,13]. In brief, the task procedure consists of two phases. In the first phase, the participants search a visual array of colored circles for a target circle colored red or green and indicate by button press the orientation (horizontal or vertical) of a bar within the target circle (Fig. 1A). Correct responses yield a probabilistic monetary reward. One of the two target colors yields “high” reward value () upon correct response in 80% of trials and in the remaining 20%, while the other yields “low” reward value () upon correct response in 80% of trials and in the remaining 20%. Incorrect or omitted responses yield no reward (). The assignment of target colors to high or low reward is counterbalanced across the sample. The second, testing, phase occurred during fMRI scanning. In this phase participants are instructed to disregard shape color and search for the odd shape in the presented array, again indicating by button press the bar orientation within the target. No feedback is provided. Half of the trials include non-target red or green shapes, serving as reward-conditioned distractors. We quantified AB via accuracy and reaction time (RT) differences between trial types (e.g., presence of previously high or low-reward distractor, no distractor present) with slower RTs and/or lower accuracy reflecting greater AB.
Fig. 1. Attentional bias measured by reward-driven attentional bias (RDAB) task.
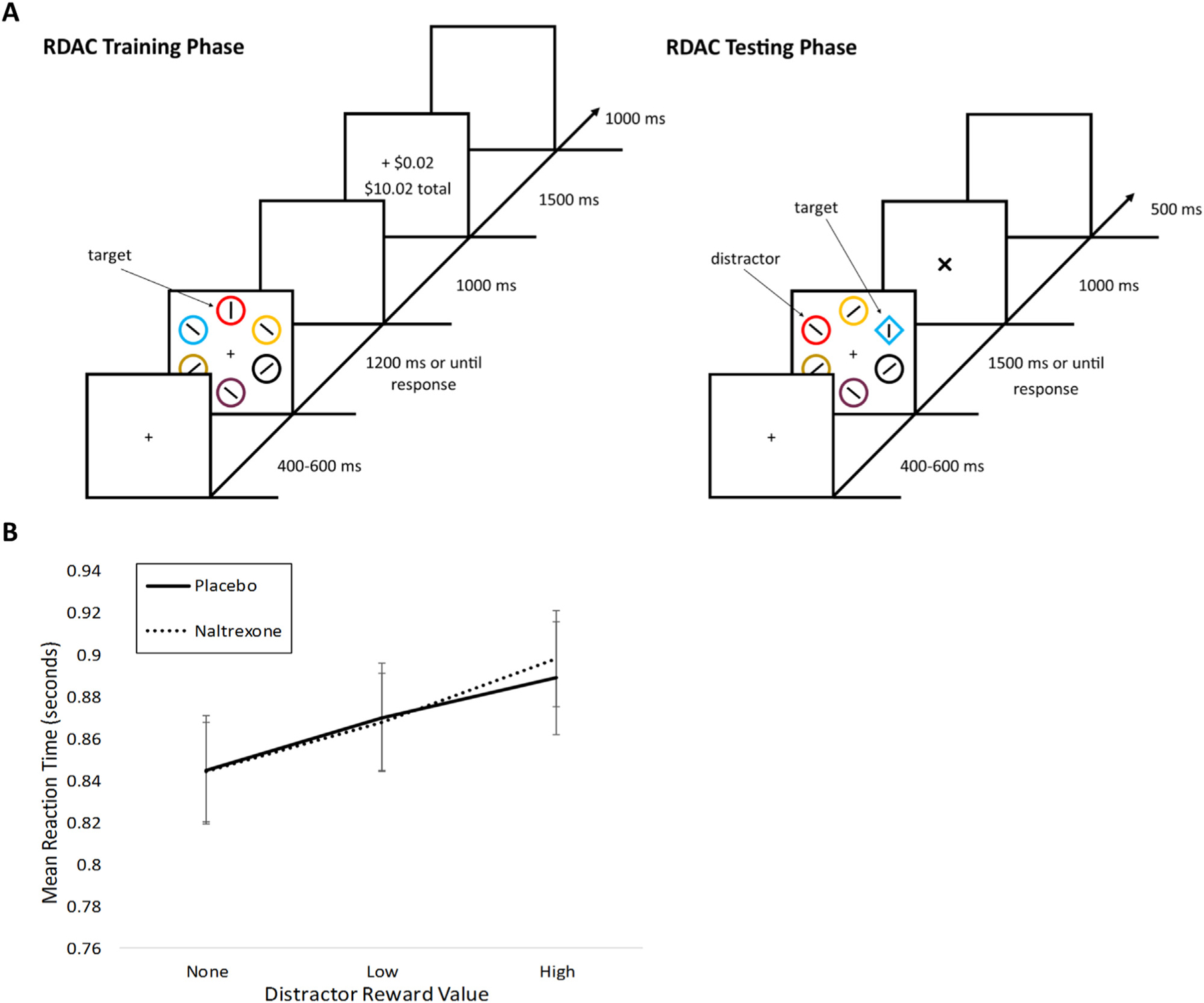
A) In the training phase, participants searched a visual array of colored circles for a target circle colored red or green and indicated by button press the orientation (horizontal or vertical) of a bar within the target circle. Correct responses yielded a probabilistic monetary reward. Incorrect or omitted responses yielded no reward (0¢). The testing phase occurred during fMRI scanning. In this phase, participants were instructed to disregard shape color and search for the odd shape in the presented array, again indicated by button press the bar orientation within the target. No feedback was provided. Half of the trials included non-target red or green shapes, serving as reward-conditioned distractors. B) Mean reaction time (seconds) are shown for testing trials in which no, low, or high reward distractors were present and for placebo versus naltrexone visits. Visit type (placebo versus naltrexone) is indicated by line type.
Alcohol ab behavioral tasks
Participants also completed two tasks designed to measure AB toward alcohol-related cues. These tasks were completed on a laptop computer in a testing room within the imaging facility.
We quantified extended attentional hold with a previously described modified attentional blink task [13]. The task consists of four blocks of 48 trials each, and each trial begins with an 18 pt white fixation cross followed by a series of 17 greyscale photograph images () displayed for 100 ms each with a 0 ms inter-stimulus-interval. Images in each stream were neutral, upright landscape or house photographs, excluding a distractor and a target image. Half of distractor images were neutral (kitchen-related) while the other half were alcohol-related images. Alcohol-related stimuli were randomly drawn from a set of 59 images. Target images were house images that were rotated 90° to the left or right. The target image appeared in the stream either two (lag2) or eight (lag8) images after the distractor image. At the end of the stimuli presentation stream, a response screen prompted participants to indicate the direction of the target image rotation (left or right) via keypress. AB was assessed via mean accuracy in reporting the correct target image rotation for each task condition (alcohol or neutral distractor).
We quantified selective attention capture by alcohol-related cues with a previously described dot-probe task [13,48,49]. The task procedure consisted of 56 total trials, and each trial began with a white fixation cross followed by the presentation of two greyscale images on the left and right-hand sides of the cross. Images were presented for 150 ms, followed by a 50 ms inter-stimulus-interval, and the presentation of the target (a 36 pt white asterisk) for 200 ms in one of the image locations. Participants were instructed to respond to the target’s location via keypress. Each trial contained an image depicting alcohol-related content and an image depicting neutral, kitchen-related content drawn from sets of 20, and images were matched with regard to basic visual properties. Stimuli were not repeated within the task, but were among the same set as used for the attentional blink task. Left/right positioning of the alcohol-related image was pseudorandomly ordered with a ratio of 1:1. Congruent trials were defined as those in which the alcohol image was presented on the same side of the screen as the consequent target, while incongruent trials were those in which the target was presented on the opposite side as the alcohol image had been prior. AB was assessed via mean RTs collected on the different task conditions (target congruent or incongruent with the location of the alcohol-related cue).
Magnetic resonance imaging (MRI) data acquisition
We acquired fMRI data during the Reward Task test phase as T2∗-weighted echo-planar images (EPI) on a Siemens 3T Prisma MRI scanner with a 32-channel head coil (TR = 2 s, TE = 25 ms, flip angle = 50°, , voxel with a 0.5 mm gap, , 35 slices acquired at a 30° angle from the horizontal plane, 243 frames) to detect blood oxygen level-dependent (BOLD) contrast. T1-weighted high-resolution magnetization-prepared rapid gradient-echo (MPRAGE) structural images were also acquired for alignment and tissue segmentation. The following parameters were used: TR=2530 ms TE=2.27, flip angle = 9°, , 512 slices, final resolution=.
MRI data preprocessing
fMRI data were preprocessed using Analysis of Functional Neuroimages (AFNI, version 16.0.1 software [50]. Preprocessing steps included slice time correction, reorienting of oblique slices to the axial plane, image realignment to correct for head motion, editing of outlier time points (3dDespike), nuisance variable regression (white matter and cerebral spinal fluid signals as well as six motion covariates), linear detrending, spatial smoothing with a 5 mm full-width half-maximum Gaussian kernel, and scaling to percent signal change.
Whole-brain analysis
A whole-brain, voxel-wise analysis sought to identify brain regions that were activated in response to the presence of reward-conditioned distractors, as well as regions that were significantly altered by acute naltrexone administration. Using a combination of SPM12 and MAT-LAB, we created a multiple regression design matrix that modeled event type by distractor value (high reward, low reward, no reward), shape (circle or diamond), and response (correct, incorrect, missed). Missed trials were defined as those in which no response was recorded. Event types were time-locked to onset of the task frame and convolved with a canonical hemodynamic response function. In addition, to test for differences in BOLD signal between conditions that are independent of RT differences, the design matrix included a parametric modulation regressor of trial-specific RT for each experimental condition [51]. Six head-movement parameters derived from realignment in preprocessing were also included, as well as a variable number of motion-regressors based upon excessive motion (trials in which there was a frame-wise displacement of from the previous time point). A total of 25 event types were modeled (3 reward values × 2 shapes × 2 (accurate and inaccurate) × 2 (each event type with and without RT modulation)) and missed trials, which varied based upon individual accuracy and performance. We next averaged beta images from our first-level analysis across distractor shape to create average images for each reward value and pill condition in accurate trials only. Therefore, we calculated six average beta images for each participant: high reward naltrexone, low reward naltrexone, no reward naltrexone, high reward placebo, low reward placebo, no reward placebo. These parameter estimates were entered into a pill (2) × reward (3) within-subject ANOVA at the second (group) level using the flexible factorial function of SPM12. Session order and subject means were included as additional factors in the model and contrasts investigating the main effect of pill (Naltrexone > Placebo), main effect of reward (high and low reward trials > no reward trials), and interaction between pill and reward were conducted. A voxel-wise family-wise-error (FWE) correction with using Gaussian random field theory implemented in SPM was applied. To eliminate spurious voxel activations, we applied an additional spatial extent threshold of 5 voxels.
Region of interest (ROI) analysis
To more explicitly test the hypothesis that naltrexone alters brain reward processing, we also carried out analyses within a pre-defined reward network including regions known to be involved in reward-related processes [52]. Anatomical ROIs for reward-related structures (Fig. 3) included bilateral amygdala, caudate, putamen, pallidum, frontal medial orbitofrontal cortex (OFC; defined from the Automated Anatomical Labeling (AAL) atlas [53]), the ventral tegmental area (VTA) [54], and the nucleus accumbens (NAcc) [55]. For the NAcc ROI, 8 mm spheres were built around the MNI coordinates , , (left NAcc) and , , mm (right NAcc) and were treated as a single bilateral ROI. For each ROI, mean parameter estimates (beta values) for each trial distractor type (high reward, low reward, none) were extracted for both naltrexone and placebo conditions. These estimates were entered into a Multivariate Analysis of Variance (MANOVA) using the MIXED procedure in SAS Software to test for effects of pill and distractor type on the activation of the reward network.
Fig. 3. Anatomical regions of interest included in reward network.
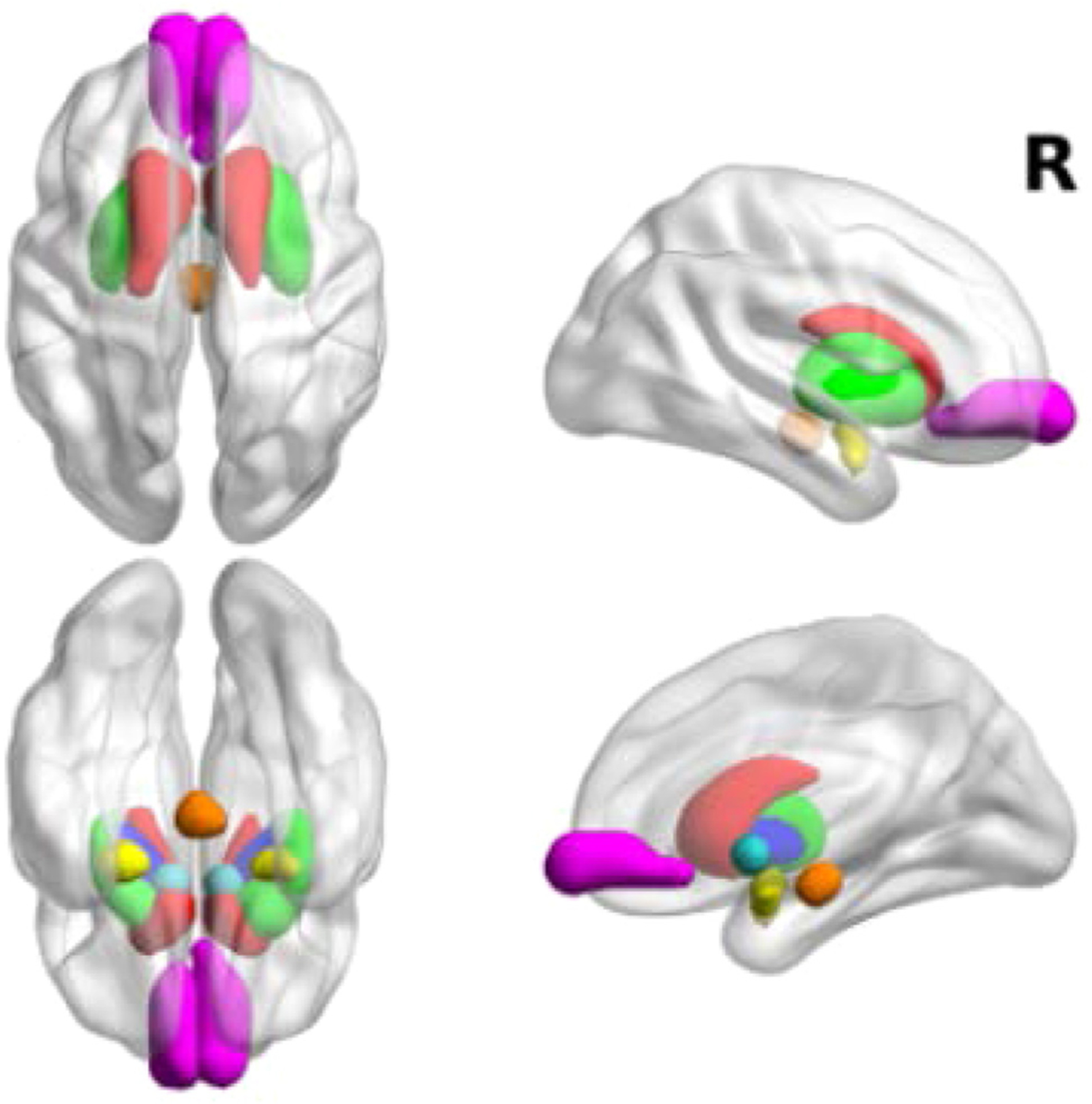
Anatomical reward-related structures included in the network analysis are shown: bilateral amygdala (yellow), caudate (red), putamen (green), pallidum (blue), frontal medial orbitofrontocortex (magenta), ventral tegmental area (orange), and nucleus accumbens (cyan).
Behavioral analysis
The primary behavioral measure of interest in the reward conditioning task was RT difference between trials with a previously-rewarded distractor present or absent. To this end, mean RTs were calculated across the different reward conditions, defined as trials in which either a high-value (), low-value (), or no distractor () was present. We excluded the first trial, incorrect trials, and trials with RTs that were <200 ms or >3 standard deviations away from the participant’s mean RT. We entered RTs into a repeated measures (RM) ANOVA to test for pill × reward level interactions.
For the modified attentional blink task, mean accuracy of response to targets was calculated for each task condition (lag 2 or 8; kitchen or alcohol-related distractor). Lower accuracy at lag 2 represents greater AB or extended attentional hold by the critical distractor. Trials in which the RT was <200 ms were excluded from mean calculations. Mean accuracy was entered into a RM ANOVA to test for effects of pill (naltrexone/placebo), lag (8/2), and distractor (alcohol/neutral), and their interactions on task accuracy.
In the spatial cuing (dot-probe) task, mean RT was calculated across task conditions (target spatially congruent/incongruent to alcohol cue). Faster RT in trials in which the cue and the target are congruent represents AB toward the cue. RTs from incorrect trials, trials in which the RT was <200 ms, or >2 standard deviations away from the individual’s mean RT were excluded from analyses. RTs were entered into a RM ANOVA to assess the effects of pill and congruency on task RTs.
Brain-Behavior correlations
We sought to investigate to what degree reward-related brain activation within the reward network was associated with reward-related behavior in the task. To this end, we carried out a multivariate multiple regression using a mixed model in SAS. The effect of naltrexone on reward-related activation () for each region of the reward network were entered as dependent variables while age, session order, and were entered as explanatory variables.
To explore brain-behavior correlations among individual ROIs within the reward network, a single measure capturing the effect of naltrexone on reward activation was calculated according to the following equation:
A similar behavioral measure was defined and calculated as:
These measures were correlated using a partial correlation in SPSS that controlled for session order and age.
Results
Demographic and psychometric data
Our sample consisted of healthy, young adult males reporting a wide range of alcohol use. Binge scores ranged from 1 to 86 with a mean of 17, while drinks per week ranged from 0 to 40 with a mean of 7.0. Additional psychometric and substance abuse measures are reported in Table 1.
Behavioral task analyses
A repeated-measures ANOVA of RTs in the reward task with the factors of pill and distractor reward value revealed a significant main effect of reward (, p<0.001; Fig. 1B), supporting the effectiveness of the task in eliciting reward-related attentional bias. However, there was not a main effect of pill (, p = 0.87) nor a pill-by-reward interaction (, p = 0.80). To explore whether these effects may be influenced by drinking level, we added drinking group as a between-subjects factor. There were no interactions of drinking group with reward (, p = 0.48), pill (, p = 0.65), or the pill-by-reward interaction (, p = 0.58).
A repeated-measures ANOVA of accuracy in the attentional blink task with the factors of pill, lag, and distractor type revealed a significant main effect of lag (, p<0.001), as well as an interaction between lag and distractor type (i.e., lag 8 versus lag 2 for alcohol versus neutral) (F(1,22)=5.81, p = 0.025), consistent with the ability of this task to capture alcohol AB. There was no main effect of pill (, p = 0.60), and there was no interaction of pill with lag (, p = 0.33), or distractor type (, p = 0.63), or an interaction between the three factors (, p = 0.07). However, a post-hoc examination of this nonsignificant trend for the interaction of pill, lag, and distractor type indicated a tendency for naltrexone to reduce the alcohol blink bias with a large effect size (). Exploratory analyses of drinking level effects did not find significant interactions of drinking group with lag (F(1,21)=0.08, p = 0.78), distractor type (F(1,21)=0.60, p = 0.45), pill (F(1,21)=0.72, p = 0.40), the lag-by-distractor type interaction (F(1,21)=0.11, p = 0.75), or the full interaction between all factors (F(1,21)=0.68, p = 0.42),
A repeated-measures ANOVA of RTs in the spatial cuing task with the factors of pill and target congruency revealed a significant main effect of congruency (, p = 0.005), indicating significant AB towards alcohol cues. However, there was no main effect of pill (, p = 0.90) or a pill-by-congruency interaction (, p = 0.23). A post-hoc examination of this nonsignificant trend for the interaction of pill and congruency indicated a tendency for naltrexone to reduce the RT bias towards alcohol cues with a medium effect size (). Again, exploratory analyses of drinking level effects did not find significant interactions of drinking group with congruency (F(1,20)=0.48, p = 0.50), pill (F(1,20)=0.02, p = 0.89), or their interaction (F(1,20)=0.60, p = 0.45).
Whole brain analysis
To test for the effects of a reward-conditioned distractor and pill on brain activation, we used a ANOVA [pill × distractor (no, low reward, high reward)], comparing parameter estimates from trials of each reward level in the naltrexone and placebo sessions. The contrast of the main effect of pill carried out across all voxels revealed significant clusters after family-wise error correction (p<0.05 corrected, ) in ten brain regions, summarized in Table 2 and visualized in Fig. 2. These clusters comprise regions implicated in aspects of visuomotor control, which is an essential component of the visual search task employed here. No clusters survived the family-wise error correction for the contrast of the main effect of reward, nor for the interaction of pill × reward.
Table 2.
Differences in BOLD responses associated with Naltrexone versus Placebo conditions.
| Cluster size (k) | Peak Region |
MNI coordinates
|
t-score | p-value (FWE) | Cohen’s d | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive Regions | |||||||
| 25 | L Caudate | −12 | 1 | 13 | 7.26 | 0.000 | 1.67 |
| −1 | −2 | −1 | 5.87 | 0.001 | 1.35 | ||
| 18 | R Caudate | 9 | 8 | 3 | 6.68 | 0.000 | 1.53 |
| 13 | 5 | 13 | 5.82 | 0.002 | 1.34 | ||
| 10 | R Thalamus | 9 | −13 | 7 | 6.41 | 0.000 | 1.47 |
| 16 | −2 | 20 | 5.12 | 0.030 | 1.17 | ||
| 12 | R Cerebellum | 30 | −41 | −32 | 6.36 | 0.000 | 1.46 |
| 34 | −48 | −36 | 5.85 | 0.001 | 1.34 | ||
| 15 | R Sensorimotor | 44 | −23 | 41 | 6.33 | 0.000 | 1.45 |
| x | R Cerebellum | 20 | −72 | −22 | 6.18 | 0.000 | 1.42 |
| 16 | −72 | −36 | 5.41 | 0.009 | 1.24 | ||
| 5 | R Intraparietal Sulcus | 37 | −58 | 34 | 5.45 | 0.008 | 1.25 |
| 44 | −55 | 38 | 5.38 | 0.010 | 1.23 | ||
| Negative Regions | |||||||
| 7 | L Cuneus | −19 | −69 | 24 | −5.45 | 0.008 | 1.25 |
| 6 | R Frontal Eye Field | 34 | −9 | 52 | −5.69 | 0.003 | 1.31 |
| 5 | L Primary Motor Cortex | −29 | −23 | 62 | −5.65 | 0.003 | 1.3 |
Fig. 2. Contrast of the main effect of naltrexone carried out across all voxels.
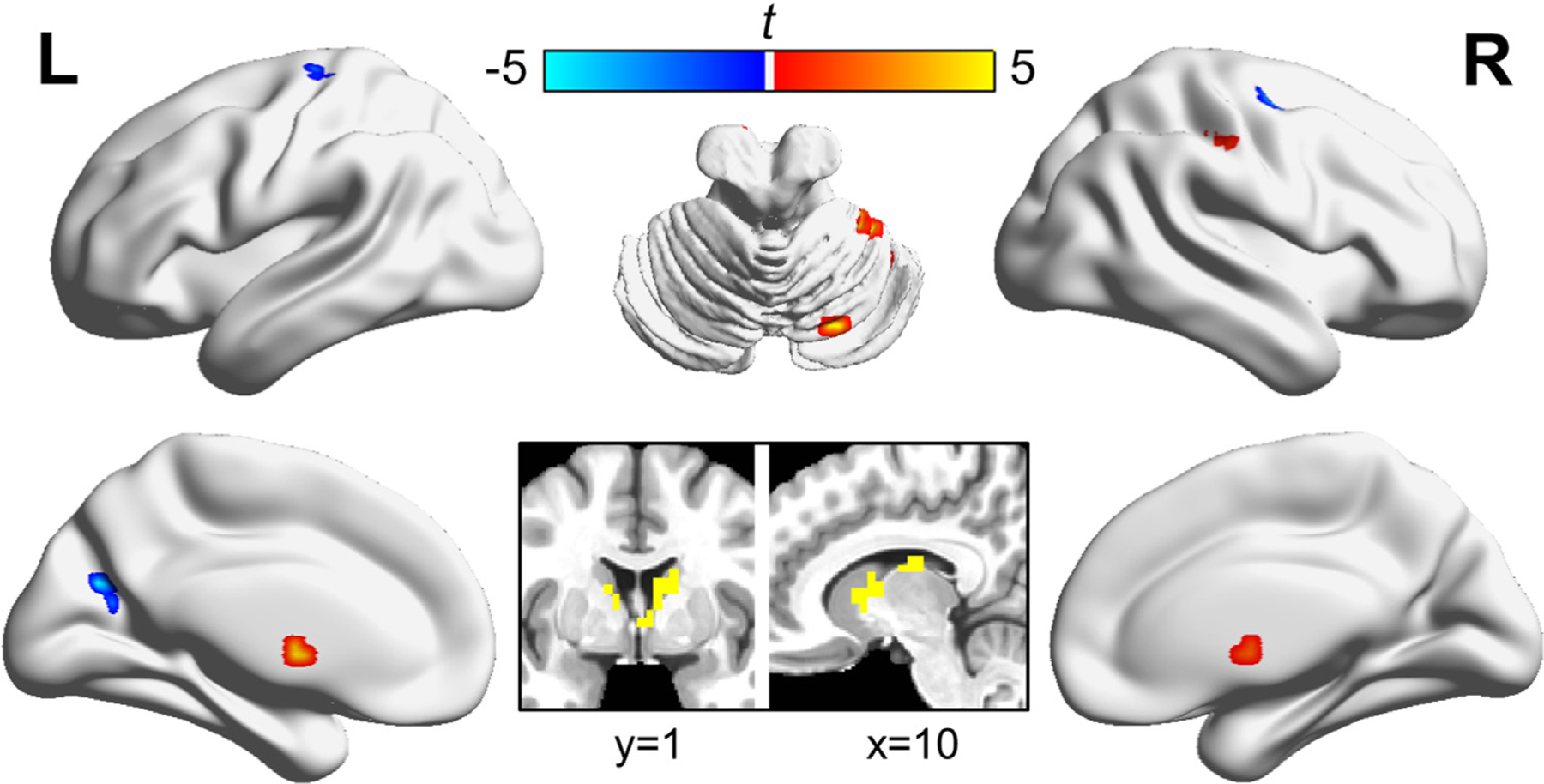
Results for the main effect of pill type (naltrexone versus placebo) from the whole-brain ANOVA analysis are shown. Significant clusters (after family-wise error correction of p<0.05, ) were identified in ten highlighted brain regions. Information regarding the peaks can be found in Table 2.
ROI analysis
To test the effects of pill and reward level on 12 components of the reward network, parameter estimates from each of the pre-defined ROI (Fig. 3) were entered into a MANOVA model. There was a significant main effect of pill on the overall reward network (F(12, 1068)=7.33, p<0.0001). However, we did not detect a significant main effect of reward (F(24, 1068)=1.31, p = 0.15), nor a pill-by-reward interaction (F(24, 1068)=0.81, p = 0.72). We further tested which ROIs within the reward network contributed to the main effect of pill. Six ROI within the network revealed significant main effects of pill after a False Discovery Rate (FDR) correction for multiple comparisons [56] including the caudate, putamen, nucleus accumbens, and pallidum (Fig. 4).
Fig. 4. Effect of naltrexone on reward-related structure BOLD activation.
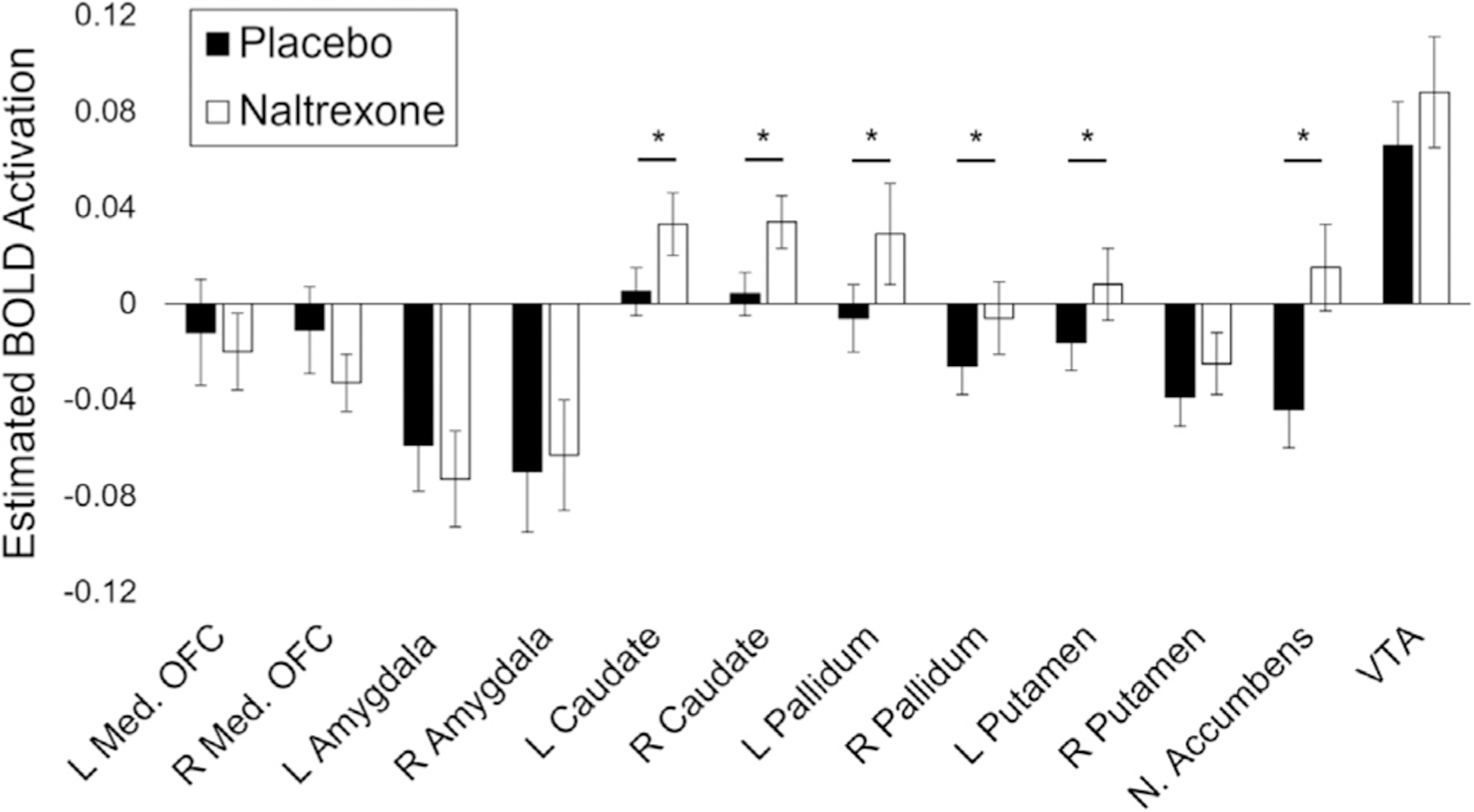
BOLD activation means for naltrexone and placebo visits are shown for each of the 12 reward-related structures included in the reward network. Bar shading indicates visit type. Error bars represent standard errors of the mean. Asterisks (∗) denote a significant effect of naltrexone on BOLD activation as determined by univariate ANOVAs carried out independently for each region of interest.
Exploratory tests of the effects of drinking group on these relationships indicated no effect of group on reward (F(24, 108)=0.44, p = 0.99) or the pill-by-reward interaction (F(24, 108)=0.60, p = 0.93). There was a significant interaction effect of drinking group and pill on the overall reward network activation (F(24, 1068)=3.00, p<0.001). However, none of the post-hoc tests of individual ROIs survived a multiple comparison correction. The activation estimates of each ROI by pill and group are displayed in Supplemental Figure 1.
Brain-behavior correlations
Although behavioral and fMRI analyses did not suggest significant influences of naltrexone on AB to conditioned rewards (i.e., pill-by-reward interactions), it is possible that there were individual differences in naltrexone effects on the brain that account for individual differences in behavioral effects. To evaluate this possibility, we tested the relationship between reward network activation and AB to reward cues. Based upon a multivariate analysis including all sessions and subjects, we did not find a statistically significant relationship between reward network activation, which tests all ROIs simultaneously, and reward distractor effects on RTs (F(4, 12) = 1.47, p = 0.38; Wilk’s Lambda = 0.185). Although the multivariate analysis was not significant, suggesting naltrexone does not modify reward-associated brain regions involved in attentional bias for reward-conditioned cues, we sought to further rule out this possibility in an alternative approach. To further explore the effects of naltrexone on individual ROIs and AB behavior, we conducted partial correlations between the effect of naltrexone on reward activation () for each ROI and the effect of naltrexone on AB to reward cues (), controlling for session order and age. Here, we detected significant correlations between behavioral effects and brain responses to reward in two ROIs after FDR correction for multiple tests (Table 3): left pallidum (, ; Fig. 5a) and right putamen (, ; Fig. 5b). Each of these ROIs showed the same pattern of greater BOLD response when a reward-conditioned distractor was present in the naltrexone session being associated with reduced attentional bias towards the reward-conditioned cue.
Table 3.
Partial correlations between reward bias behavior and brain responses to reward.
| Region | r | P | p∗ |
|---|---|---|---|
| L Medial OFC | 0.30 | 0.24 | 0.326 |
| R Medial OFC | 0.31 | 0.23 | 0.326 |
| L Amygdala | −0.34 | 0.18 | 0.317 |
| R Amygdala | −0.43 | 0.088 | 0.212 |
| L Caudate | −0.12 | 0.65 | 0.675 |
| R Caudate | −0.34 | 0.18 | 0.317 |
| L Pallidum | −0.68 | 0.003 | 0.031 |
| R Pallidum | −0.49 | 0.046 | 0.139 |
| L Putamen | −0.58 | 0.014 | 0.058 |
| R Putamen | −0.63 | 0.007 | 0.041 |
| Nucleus Accumbens | −0.21 | 0.43 | 0.515 |
| VTA | 0.11 | 0.68 | 0.675 |
∗ P-values corrected for false discovery rate using the Benjamini and Hochberg method.
Fig. 5.
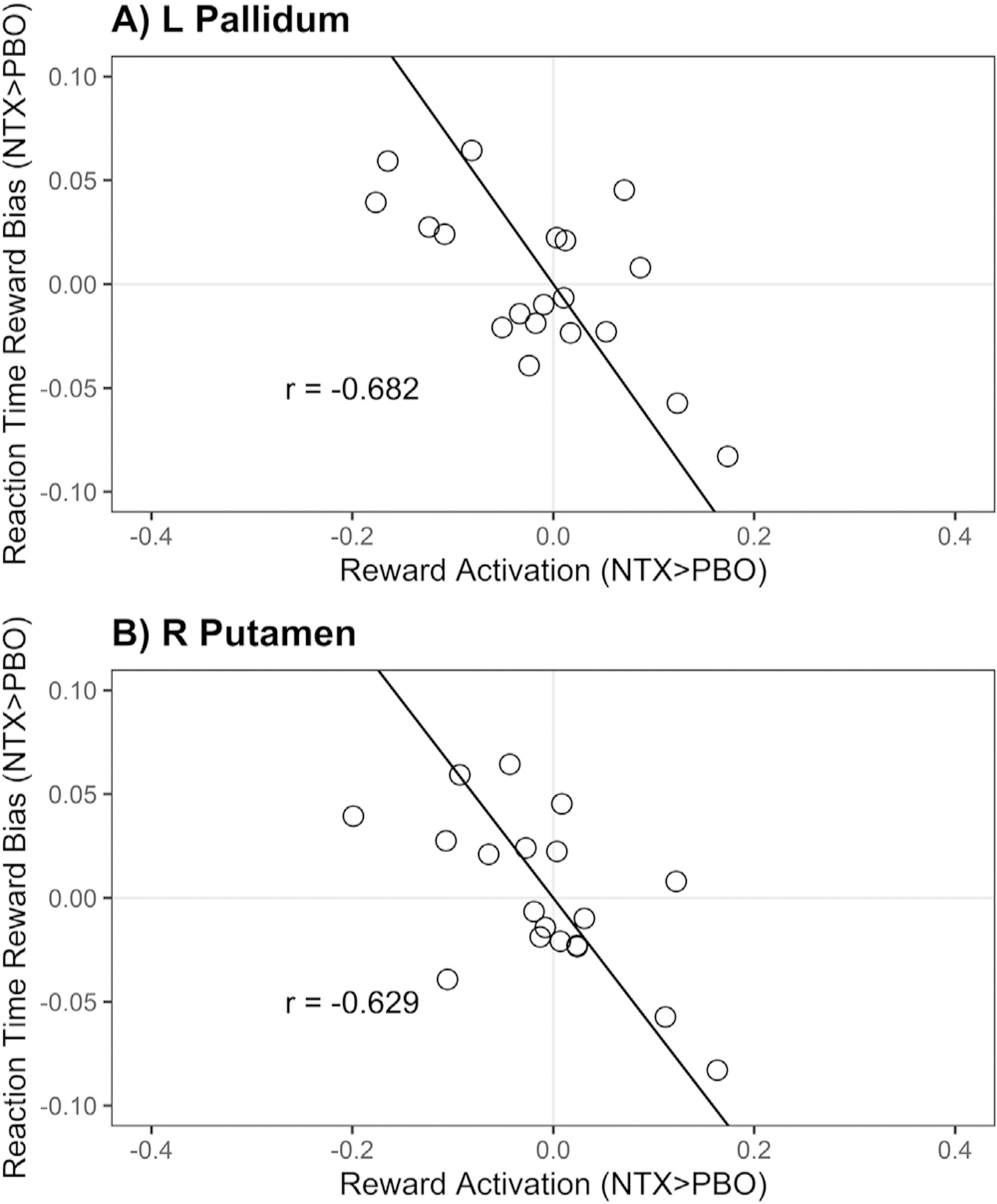
Partial correlations between behavioral effects and brain responses to reward within the reward network. Scatter plots of behavioral effects and brain responses to reward. Statistically significant relationships are displayed for A) right pallidum, B) left pallidum, C) right putamen, D) left putamen. Results from partial correlations controlling for age and session order are listed on each panel (r); results in the remaining 8 reward-related ROIs were insignificant and are shown in Table 3.
Discussion
Identifying the functional effects of naltrexone, which may contribute to its efficacy as a treatment for AUD, is essential not only for better predicting who may benefit from the drug, but even more critically for directing future research into novel therapeutic treatments. Here, a whole-brain analysis in healthy young males found that naltrexone increases BOLD activations within the caudate, right thalamus, right cerebellum and several regions implicated in visuomotor control. A focused analysis of naltrexone’s effects within a pre-defined reward-related network showed increased BOLD signal within several key reward regions: the pallidum, caudate, putamen, and nucleus accumbens. Despite large drug effect sizes (Table 2), naltrexone’s effects on BOLD activation were not influenced by the presence of reward distractor stimuli. Overall, this finding indicates that although naltrexone targets brain reward regions, drug-related effects in these regions did not consistently influence reward-driven AB in this sample, consistent with the behavioral findings of null effects of naltrexone on AB. Notably, although AB to alcohol cues may reflect a variable combination of the subjective rewarding or aversive properties of alcohol stimuli, naltrexone was similarly ineffective in reducing alcohol AB in this sample.
Although we identified naltrexone-related changes to a brain reward network, the direction of those changes apparently contrasts with previous work. Here, we found naltrexone to be associated with higher activity in the nucleus accumbens, while many previous imaging studies have shown naltrexone to reduce activations within the ventral striatum in response to alcohol-related cue presentation [15,57–60]. However, the paradigms used to provoke cue reactivity from these studies may not be directly comparable to our task activations, as the goal in the current task required diverting attention away from the reward-related cues. A somewhat surprising result is the lack of a main effect of reward on brain activity; however it’s important to note that Anderson et al. [61] demonstrated that this task primarily elicits activation in the caudate tail and visual brain regions, highlighting the differences between this task and tasks of cue-related reactivity that target nucleus accumbens. These results suggest that the RDAB task used here may invoke reward responding and processing distinct from that characterized by the previously mentioned imaging studies, although it is also possible that this lack of reward effect reflects a lack of statistical power. We would expect that imaging during the training phase of this task would indeed demonstrate a main effect of reward, as the reward cue is only present at the location of the target. By measuring brain activity during the testing phase of the task, the reward cue is only present as a distractor, so contrasting reward-cue present/absent also contrasts distractor present/absent. Correlations between greater task activation of particular regions of the reward network with reduced reward-related AB further supports the notion that naltrexone-related activation reported here is related to the unique demands of the RDAB task.
Despite the absence of naltrexone-by-reward interactions in our group-level fMRI analyses, there was individual variation in naltrexone effects on task performance, which might correspond with the known variation in naltrexone’s effectiveness. Exploiting this individual variation, brain-behavior correlations showed that naltrexone-related increases in reward-specific activations in the pallidum and putamen were associated with greater reduction in RT bias to previously rewarded distractors. These correlations indicate that increased activity in these areas, specific to naltrexone administration and the presence of rewarded distractors, may underlie behavioral changes in AB to reward-conditioned cues. Recent work by de Laat and colleagues (2019) observed higher baseline kappa-opioid receptor (KOR) availability in the pallidum and striatum to be correlated with smaller reductions in drinking during naltrexone treatment, indicating that KORs in these regions may play a significant role in mediating naltrexone’s KOR antagonist effects [62]. Notably, de Laat and colleagues’ sample consisted of non-treatment seeking, alcohol-dependent individuals, who have lower KOR availability than healthy controls in areas including the pallidum [63]. Individuals in our sample encompassed a wide range of drinking behaviors, but were not exclusively alcohol-dependent, raising the possibility that our sample had differences in KOR availability as compared to an alcohol-dependent cohort, potentially affecting the impact of naltrexone on both the brain and behavior. This distinction is also relevant to the fMRI studies described above that reported reduced cue-induced activation in the ventral striatum on naltrexone, as these were also conducted in alcohol-dependent samples [57,58,60]. While the mu-opioid receptor has long been the focus of naltrexone studies, our findings lend support to the idea that naltrexone may exert its therapeutic effects through KORs as well [28]. Given the potentially divergent effects of mu- and kappa-opioid systems on reward circuitry [64], and findings of increased mu-receptor availability in alcohol-dependent individuals as compared to healthy controls in areas such as the ventral striatum, caudate, putamen, and thalamus [65], the interplay between these two systems must be investigated in future research into naltrexone’s effects.
Our behavioral analyses showed significant RT slowing in the presence of reward-conditioned distractors for both placebo and naltrexone sessions, demonstrating that the RDAC task elicited the expected AB behavior [66]. Yet, in contrast to previous findings that naltrexone decreases cue-reactivity to general reward stimuli [17], we found that naltrexone did not reduce AB to general rewards. One explanation for this discrepancy is the differing contexts in which the reward cues are presented in these studies: passive exposure versus irrelevant distractor. While we did find that naltrexone acutely modulated activity in the nucleus accumbens, caudate, and pallidum, activity changes in the pallidum and putamen predicted the reduction of AB to reward-conditioned cues. These modulations suggest that naltrexone’s effects on AB primarily reflect not reward processing per se, but rather top-down control of attention [27,28]. That interpretation is also consistent with the findings from our whole-brain analysis that naltrexone acutely altered activity in areas associated with visuomotor control.
Limitations
Although the effects of naltrexone on ROI associated with reward processing were highly robust, the null findings we reported should be interpreted with consideration of the small sample size. Importantly, we did not consider genetic variables in the current analysis, which have the potential to impact both drinking behaviors and neurobiological characteristics (e.g., receptor levels), with previous studies finding genetic variation to underlie individual differences in the effects of naltrexone on reward bias [67]. Another limitation was the use of a single-sex sample, introducing the possibility that our results may not generalize to females. Furthermore, this sample encompassed a spectrum of drinking behavior, and the inclusion of non-hazardous drinkers may have contributed to the null findings of the effects of naltrexone on AB. However, exploratory analyses did not suggest that naltrexone effects were larger in individuals reporting heavier binge drinking. Nonetheless, our results may not fully translate to individuals with AUD. Moreover, while naltrexone may be used therapeutically on an “as needed” basis [68,69], more typical therapeutic use entails daily dosing over a longer period of time. Finally, the lack of clinical outcome measures limits our ability to translate observed alterations in brain activity or laboratory behavior to naltrexone’s therapeutic effects. Incorporating such measures into a clinic trial of naltrexone would enable a full examination of these relationships.
Conclusions
Here we demonstrate effects of naltrexone on brain activation during a task in which participants are instructed to ignore reward-conditioned distractors. Across the sample, naltrexone altered activity in visuomotor control circuitry as well as regions associated with reward processing, but did not reduce AB or brain activation indices of AB. However, brain-behavior relationships indicated that naltrexone-induced increases in activation of the putamen and pallidum predicted reductions in AB across individuals. These results support the existing literature suggesting that naltrexone targets brain regions involved in reward processing and cognitive control, and that its effect on behavior varies substantially across individuals.
Supplementary Material
Footnotes
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.addicn.2023.100085.
Data availability
Data will be made available on request.
References
- [1].Townshend JM, Duka T, Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers, Psychopharmacology 157 (1) (2001) 67–74. [DOI] [PubMed] [Google Scholar]
- [2].Attwood AS, O’Sullivan H, Leonards U, Mackintosh B, Munafó MR, Attentional bias training and cue reactivity in cigarette smokers, Addiction 103 (11) (2008) 1875–1882. [DOI] [PubMed] [Google Scholar]
- [3].Bearre L, Sturt P, Bruce G, Jones BT, Heroin-related attentional bias and monthly frequency of heroin use are positively associated in attenders of a harm reduction service, Addict. Behav 32 (4) (2007) 784–792. [DOI] [PubMed] [Google Scholar]
- [4].Copersino ML, Serper MR, Vadhan N, et al. , Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients, Psychiatry Res 128 (3) (2004) 209–218. [DOI] [PubMed] [Google Scholar]
- [5].Manchery L, Yarmush DE, Luehring-Jones P, Erblich J, Attentional bias to alcohol stimuli predicts elevated cue-induced craving in young adult social drinkers, Addict. Behav 70 (2017) 14–17. [DOI] [PubMed] [Google Scholar]
- [6].Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM, Attentional bias predicts heroin relapse following treatment, Addiction 101 (9) (2006) 1306–1312. [DOI] [PubMed] [Google Scholar]
- [7].Nikolaou K, Field M, Duka T, Alcohol-related cues reduce cognitive control in social drinkers, Behav. Pharmacol 24 (1) (2013) 29–36. [DOI] [PubMed] [Google Scholar]
- [8].Cox WM, Hogan LM, Kristian MR, Race JH, Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome, Drug Alcohol Depend 68 (3) (2002) 237–243. [DOI] [PubMed] [Google Scholar]
- [9].Field M, Eastwood B, Experimental manipulation of attentional bias increases the motivation to drink alcohol, Psychopharmacology 183 (3) (2005) 350–357. [DOI] [PubMed] [Google Scholar]
- [10].Goldstein RZ, Volkow ND, Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications, Nat. Rev 12 (11) (2011) 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Anderson BA, Faulkner ML, Rilee JJ, Yantis S, Marvel CL, Attentional bias for nondrug reward is magnified in addiction, Exp. Clin. Psychopharmacol 21 (6) (2013) 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bjork JM, Smith AR, Chen G, Hommer DW, Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: effort anticipation, reward anticipation and reward delivery, Hum. Brain Mapp 33 (9) (2012) 2174–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Elton A, Faulkner ML, Robinson DL, Boettiger CA, Acute depletion of dopamine precursors in the human brain: effects on functional connectivity and alcohol attentional bias, Neuropsychopharmacology (2021) 1–11. [DOI] [PMC free article] [PubMed]
- [14].Spanagel R, Weiss F, The dopamine hypothesis of reward: past and current status, Trends Neurosci 22 (11) (1999) 521–527. [DOI] [PubMed] [Google Scholar]
- [15].Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K, Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people, Arch. Gen. Psychiatry 65 (4) (2008) 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mann K, Vollstadt-Klein S, Reinhard I, et al. , Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging, Alcohol. Clin. Exp. Res 38 (11) (2014) 2754–2762. [DOI] [PubMed] [Google Scholar]
- [17].Weber SC, Beck-Schimmer B, Kajdi ME, Muller D, Tobler PN, Quednow BB, Dopamine D2/3- and mu-opioid receptor antagonists reduce cue-induced responding and reward impulsivity in humans, Transl. Psychiatry 6 (7) (2016) e850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Garbutt JC, Greenblatt AM, West SL, et al. , Clinical and biological moderators of response to naltrexone in alcohol dependence: a systematic review of the evidence, Addiction 109 (8) (2014) 1274–1284. [DOI] [PubMed] [Google Scholar]
- [19].Ray LA, Chin PF, Miotto K, Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics, CNS Neurol. Disord. Drug Targets 9 (1) (2010) 13–22. [DOI] [PubMed] [Google Scholar]
- [20].Ray LA, Meredith LR, Kiluk BD, Walthers J, Carroll KM, Magill M, Combined pharmacotherapy and cognitive behavioral therapy for adults with alcohol or substance use disorders: a systematic review and meta-analysis, JAMA Netw. Open 3 (6) (2020) e208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F, Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone, Psychopharmacology 168 (1–2) (2003) 208–215. [DOI] [PubMed] [Google Scholar]
- [22].Ciccocioppo R, Martin-Fardon R, Weiss F, Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats, Neuropsychopharmacology 27 (3) (2002) 391–399. [DOI] [PubMed] [Google Scholar]
- [23].Liu N, Li B, Sun N, Ma Y, Effects of addiction-associated and affective stimuli on the attentional blink in a sample of abstinent opiate dependent patients, J. Psychopharmacol 22 (1) (2008) 64–70. [DOI] [PubMed] [Google Scholar]
- [24].Liu X, Palmatier MI, Caggiula AR, et al. , Naltrexone attenuation of conditioned but not primary reinforcement of nicotine in rats, Psychopharmacology 202 (4) (2009) 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Monti PM, Rohsenow DJ, Hutchison KE, et al. , Naltrexone’s effect on cue-elicited craving among alcoholics in treatment, Alcohol. Clin. Exp. Res 23 (8) (1999) 1386–1394. [PubMed] [Google Scholar]
- [26].Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB, Naltrexone’s effects on reactivity to alcohol cues among alcoholic men, J. Abnorm. Psychol 109 (4) (2000) 738–742. [PubMed] [Google Scholar]
- [27].Mitchell JM, Tavares VC, Fields HL, D’Esposito M, A Boettiger C, Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls, Neuropsychopharmacology 32 (2) (2007) 439–449. [DOI] [PubMed] [Google Scholar]
- [28].Altamirano LJ, Fields HL, D’Esposito M, A Boettiger C, Interaction between family history of alcoholism and locus of control in the opioid regulation of impulsive responding under the influence of alcohol, Alcohol. Clin. Exp. Res 35 (11) (2011) 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Arnsten AF, Neville HJ, Hillyard SA, Janowsky DS, Segal DS, Naloxone increases electrophysiological measures of selective information processing in humans, J. Neurosci 4 (12) (1984) 2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arnsten AF, Segal DS, Neville HJ, et al. , Naloxone augments electrophysiological signs of selective attention in man, Nature 304 (5928) (1983) 725–727. [DOI] [PubMed] [Google Scholar]
- [31].Ray LA, Hutchison KE, MacKillop J, et al. , Effects of naltrexone during the descending limb of the blood alcohol curve, Amer. J. Addict 17 (4) (2008) 257–264. [DOI] [PubMed] [Google Scholar]
- [32].Garbutt JC, Kranzler HR, O’Malley SS, et al. , Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial, JAMA 293 (13) (2005) 1617–1625. [DOI] [PubMed] [Google Scholar]
- [33].Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, S O’Malley S, Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking, Biol. Psychiatry 62 (6) (2007) 694–697. [DOI] [PubMed] [Google Scholar]
- [34].Sheehan DV, Lecrubier Y, Sheehan KH, et al. , The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10, J. Clin. Psychiatry 59 (Suppl 20) (1998) 34–57 22–33;quiz. [PubMed] [Google Scholar]
- [35].Drinking Levels Defined. National Institute on Alcohol Abuse and Alcoholism Accessed 11/12/2020, 2020.
- [36].Boettiger CA, Kelley EA, Mitchell JM, D’Esposito M, L Fields H, Now or Later? An fMRI study of the effects of endogenous opioid blockade on a decision-making network, Pharmacol. Biochem. Behav 93 (3) (2009) 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M, Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II, Addiction 88 (6) (1993) 791–804. [DOI] [PubMed] [Google Scholar]
- [38].Patton JH, Stanford MS, Barratt ES, Factor structure of the Barratt impulsiveness scale, J. Clin. Psychol 51 (6) (1995) 768–774. [DOI] [PubMed] [Google Scholar]
- [39].Tarter RE, Kirisci L, The Drug Use Screening Inventory for adults: psychometric structure and discriminative sensitivity, Am. J. Drug Alcohol Abuse 23 (2) (1997) 207–219. [DOI] [PubMed] [Google Scholar]
- [40].Mann RE, Sobell LC, Sobell MB, Pavan D, Reliability of a family tree questionnaire for assessing family history of alcohol problems, Drug Alcohol Depend 15 (1–2) (1985) 61–67. [DOI] [PubMed] [Google Scholar]
- [41].Cservenka A, Gillespie AJ, Michael PG, Nagel BJ, Family history density of alcoholism relates to left nucleus accumbens volume in adolescent girls, J. Stud. Alcohol Drugs 76 (2015) 47–56. [PMC free article] [PubMed] [Google Scholar]
- [42].Rotter JB, Generalized expectancies for internal versus external control of reinforcement, Psychol. Monogr 80 (1) (1966) 1–28. [PubMed] [Google Scholar]
- [43].Conners CK, Erhardt D, Sparrow EP, CAARS-Self-Report: Long Version (CAARS-S:L), Multi-Health Systems, North Totawanda, NY, 1998. [Google Scholar]
- [44].Henry JD, Crawford JR, The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample, Br. J. Clinic. Psychol 44 (Pt 2) (2005) 227–239. [DOI] [PubMed] [Google Scholar]
- [45].Cooper ML, Motivations for alcohol use among adolescents: development and validation of a four-factor model, Psychol. Assess 6 (2) (1994) 117–128. [Google Scholar]
- [46].Mehrabian A, Russell JA, A questionnaire measure of habitual alcohol use, Psychol Rep 43 (3 Pt 1) (1978) 803–806. [DOI] [PubMed] [Google Scholar]
- [47].Townshend JM, Duka T, Patterns of alcohol drinking in a population of young social drinkers: a comparison of questionnaire and diary measures, Alcohol Alcohol 37 (2) (2002) 187–192. [DOI] [PubMed] [Google Scholar]
- [48].Elton A, Chanon VW, Boettiger CA, Multivariate pattern analysis of the neural correlates of smoking cue attentional bias, Pharmacol. Biochem. Behav 180 (2019) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chanon VW, Sours CR, Boettiger CA, Attentional bias toward cigarette cues in active smokers, Psychopharmacology 212 (3) (2010) 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cox RW, AFNI: software for analysis and visualization of functional magnetic resonance neuroimages, Comput. Biomed. Res 29 (3) (1996) 162–173. [DOI] [PubMed] [Google Scholar]
- [51].Taylor J, Rastle K, Davis MH, Interpreting response time effects in functional imaging studies, Neuroimage 99 (2014) 419–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liu X, Hairston J, Schrier M, Fan J, Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies, Neurosci. Biobehav. Rev 35 (5) (2011) 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. , Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain, Neuroimage 15 (1) (2002) 273–289. [DOI] [PubMed] [Google Scholar]
- [54].Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA, Resting state networks distinguish human ventral tegmental area from substantia nigra, Neuroimage 100 (2014) 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Neto LL, Oliveira E, Correia F, Ferreira AG, The human nucleus accumbens: where is it? A stereotactic, anatomical and magnetic resonance imaging study, Neuromodulatione 11 (1) (2008) 13–22. [DOI] [PubMed] [Google Scholar]
- [56].Benjamini Y, Hochberg Y, Controlling the false discovery rate: a practical and powerful approach to multiple testing, J. R. Stat. Soc. Ser. B 57 (1) (1995) 289–300. [Google Scholar]
- [57].Bach P, Weil G, Pompili E, et al. , Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone, Addict. Biol 25 (1) (2020) e12717. [DOI] [PubMed] [Google Scholar]
- [58].Lukas SE, Lowen SB, Lindsey KP, et al. , Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study, Neuroimage 78 (2013) 176–185. [DOI] [PubMed] [Google Scholar]
- [59].Mann K, Bladstrom A, Torup L, Gual A, van den Brink W, Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene, Biol. Psychiatry 73 (8) (2013) 706–713. [DOI] [PubMed] [Google Scholar]
- [60].Schacht JP, Randall PK, Latham PK, et al. , Predictors of naltrexone response in a randomized trial: reward-related brain activation, OPRM1 Genotype, and smoking status, Neuropsychopharmacology 42 (13) (2017) 2640–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Anderson BA, Laurent PA, Yantis S, Value-driven attentional priority signals in human basal ganglia and visual cortex, Brain Res 1587 (2014) 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].de Laat B, Goldberg A, Shi J, et al. , The kappa opioid receptor is associated with naltrexone-induced reduction of drinking and craving, Biol. Psychiatry 86 (11) (2019) 864–871. [DOI] [PubMed] [Google Scholar]
- [63].Vijay A, Cavallo D, Goldberg A, et al. , PET imaging reveals lower kappa opioid receptor availability in alcoholics but no effect of age, Neuropsychopharmacology 43 (13) (2018) 2539–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Spanagel R, Herz A, Shippenberg TS, Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway, Proc. Natl. Acad. Sci. U.S.A 89 (6) (1992) 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Weerts EM, Kim YK, Wand GS, et al. , Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects, Neuropsychopharmacology 33 (3) (2008) 653–665. [DOI] [PubMed] [Google Scholar]
- [66].Anderson BA, Laurent PA, Yantis S, Value-driven attentional capture, Proc. Natl. Acad. Sci. U.S.A 108 (25) (2011) 10367–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Anton RF, Oroszi G, O’Malley S, et al. , An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study, Arch. Gen. Psychiatry 65 (2) (2008) 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hernandez-Avila CA, Song C, Kuo L, Tennen H, Armeli S, Kranzler HR, Targeted versus daily naltrexone: secondary analysis of effects on average daily drinking, Alcohol. Clin. Exp. Res 30 (5) (2006) 860–865. [DOI] [PubMed] [Google Scholar]
- [69].Kranzler HR, Tennen H, Armeli S, et al. , Targeted naltrexone for problem drinkers, J. Clin. Psychopharmacol 29 (4) (2009) 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


