Introduction:
This study aimed to evaluate the clinical efficacy and safety of 4 weekly formulations of glucagon-like peptide 1 receptor agonists (GLP-1RAs) on glycemic control, including glycemic control, by using a network meta-analysis (NMA).
Methods:
PubMed, EMBASE, and Cochrane Library Central Register of Controlled Trials were searched from inception until June 10, 2022. Randomized clinical trials (RCTs) enrolling participants with diabetes mellitus type 2 and a follow-up of at least 12 weeks were included, for which 4 eligible GLP-1RAs Exenatide, Dulaglutide, Semaglutide, Loxenatide were compared with either each other or placebo. The primary outcome is the change of hemoglobin A1c level. Secondary outcomes including additional glycemic control indicators and adverse events (AE). Frequentist random-effect NMA were conducted for effect comparison. This meta-analysis was registered on PROSPERO, CRD42022342241.
Results:
The NMA synthesized evidence from 12 studies covering 6213 patients and 10 GLP-1RA regimens. A pairwise comparison of glycosylated hemoglobin type A1C (HbA1c) lowering effects showed that once-weekly GLP-1 receptor agonists were significantly better than placebo, and their glucose-lowering intensity was Semaglutide 2.0mg, Semaglutide 1.0mg, Dulaglutide 4.5mg, and Semaglutide 0.5mg, Dulaglutide 3.0mg, PEX168 200ug, Dulaglutide 1.5mg, PEX168 100ug and Dulaglutide 0.75mg. The GLP-1RA regimen has a comparable safety profile for hypoglycemia. And with the exception of PEX168, all other long-acting GLP-1RA drugs had lower rates of diarrhea, nausea and vomiting than placebo.
Conclusion:
Regimens of GLP-1RAs had differential glycemic control. The efficacy and safety of Semaglutide 2.0mg in comprehensively lowering blood sugar showed the best performance.
Keywords: glucagon-like peptide 1, HbA1c, type 2 diabetes
1. Background
Diabetes mellitus type 2 (T2DM) is the most prevalent disease worldwide and is emerging as a serious public health threat.[1] At present, China has become the country with the largest number of diabetic patients in the world. In the past 10 years (2011–2021), the number of diabetic patients in China has increased from 90 million to 140 million, a growth rate of 56%. Therefore, the means to reduce blood sugar emerge in an endless stream. Among them, glucagon-like peptide 1 receptor agonists (GLP-1RAs), as an insulin secretagogue to lower blood sugar, have an increasingly important clinical status.
Since the first GLP-1RA was approved in 2005 (Exenatide twice daily) for T2DM, the class has developed with newer compounds having more pronounced effects on glycemic control and body weight.[2] Compared with other marketed hypoglycemic drugs, GLP-1RAs not only have oral dosage forms, but also injections. It also shows superiority in lowering blood sugar and reducing body weight. Comparing the evidence for the efficacy and safety of GLP-1RAs is therefore critical for choosing an appropriate clinical pathway.[3]
Previous meta-analysis have examined endpoints between 24 and 30 weeks. They did not include long-term outcomes that may be equally important. In addition, there was no correlation between dosage and adverse reactions in previous drug safety evaluation. To fufil this important evidentiary gap, the present analysis was conducted by focusing on GLP-1RAs approved.
2. Methods
The report of the present study is in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.[4,5] This article is based on previously conducted studies which does not contain any new studies with human participants or animals. This systematic review and meta-analysis reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement and was registered at International Prospective Register of Systematic Reviews (CRD42022342241).
2.1. Study selection
We only included the randomized clinical trials (RCTs) published in English and completed, which compared various weekly preparations of GLP-RAs in subjects with T2DM. The main research focus of this study is the changes of glycosylated hemoglobin. Included studies had to report this primary endpoint in the adult population (age 18 years, male or female).
2.2. Study Identification
Search strategies for potentially eligible clinical trials were pre-specified for the PubMed, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) databases from inception until April 30, 2022. The terms and rules used for the search strategy are summarized in Table S1, Supplemental Digital Content, http://links.lww.com/MD/J192, and the studies were filtered with all 4 levels of conditions in it. The type of included literature is research paper, and the corresponding trial registration is attached to the abstract. Repeat the search process using sentence-by-sentence and capitalization.
After removal of duplicates, the title and abstracts of search results were screened for relevance by a single author. The full texts of remaining results were independently assessed in duplicate by 2 authors for inclusion based on predetermined criteria. The final list of included studies was decided on discussion between authors with full agreement required prior to inclusion. No disagreements required resolution by a third reviewer (See Table S1, Supplemental Digital Content, http://links.lww.com/MD/J192, Supplemental Content, Strategy of literature retrieval).
The studies published in English were considered eligible for inclusion if they were RCTs conducted on individuals with T2DM mellitus and compared GLP-1RAs of interest with each other or with a control group, which contained results of at least one of the prespecified primary and secondary endpoint. Eligible patients should satisfy treated with metformin at least 90 days and had a follow-up of at least 12 weeks.
2.3. Data extraction
Data were extracted using piloted forms, independently and in duplicate by 2 authors which were transcribed onto a dedicated database. Study characteristics including authors, year of publication, journal or conference name, trial name, trial registration, sponsorship, type of sponsor, RCT design (e.g., 2-arm vs 3-arm, double blind vs triple blind vs open label), follow-up duration, total sample size, and end point data were extracted. And patient characteristics including age, sex, diabetes duration, and baseline values of outcomes were also extracted. Moreover, treatment characteristics including preparation (drug, administration route, and dosage) and administration frequency, sample size of the arm, other medications and outcome data simultaneously extracted.
Two investigators independently assessed random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases to assess risk of bias according to the Cochrane Manual.
2.4. Outcomes
The pre-set primary outcome was change in hemoglobin A1c (HbA1c) from baseline to the endpoint of every study. Secondary outcomes included changes in fasting plasma glucose concentrations, body weight; and the proportion of patients reporting adverse events (AE) such as hypoglycemia, nausea, vomiting, diarrhea, and headache.
2.5. Data analysis
In this network meta-analysis (NMA), we estimated standardized mean differences (SMD) for continuous outcomes and summary odd ratios (ORs) for dichotomous outcomes. SMD or ORs and an associated 95% confidence interval (CI) were presented for the results. For the continuous outcomes HbA1c (%) fasting plasma glucose (mmol/L), and body weight (kg), a treatment associated with a greater mean reduction from baseline was favored. For dichotomous outcomes, a treatment associated with a decrease in the OR was favored, which meant the incidence of AE withdrawals and hypoglycemic episodes were low. To rank the treatments for each outcome, we used the surface under the cumulative ranking curve and the mean ranks.
The relative efficacy for each continuous outcome was represented by mean difference in the change of value from baseline to endpoint across treatment groups. The sample size and event count of each arm were used for comparison in the analyses of dichotomous outcomes, in which the relative efficacy was measured by OR. Frequentist NMA with random effects were performed for all outcomes. Along with that, I2 was still computed and reported to quantify heterogeneity across studies but not used to choose random-effects model versus fixed-effects model.
The base-case analysis were based on consistency models. Inconsistency in the analysis was tested using the “design-by-treatment interaction” approach. In all analysis, placebo was used as the reference group. For the primary outcome, pairwise comparison was also conducted.
All analysis were conducted using Stata 16. Two-sided p values of 0.05 or less were considered statistically significant.
3. Results
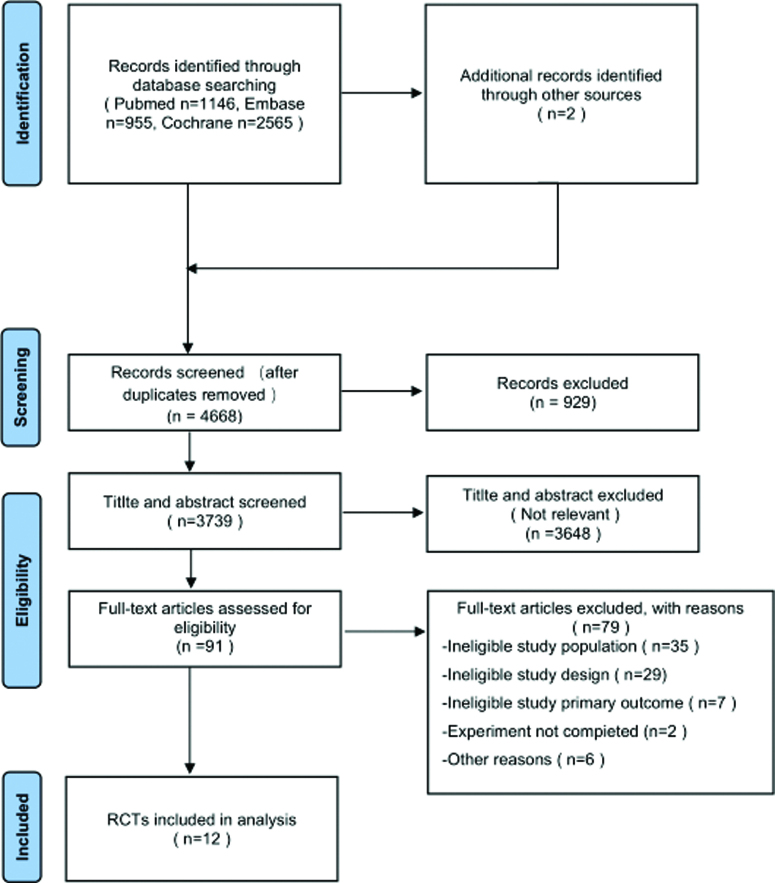
Database searches yielded 4666 records (PubMed 1146; Embase 955; Cochrane Library 2565). The specific screening flow chart is shown in Figure 1.
Figure 1.
Flow diagram of the search results and study selection.
3.1. Literature Identification
The RCTs were published from 2014 to 2020 and the characteristics of the twelve included studies are shown in Table 1[6–17] The mean age of all participants was 61 (range 50–59) and mean duration was 27.6 weeks (range 12–52 weeks). Patients had mean baseline HbA1c of 8.17% (range 7.30–8.64), mean baseline body-mass index of 30.9 kg/m² (26.0–36.0), and mean duration of diabetes of 5.6 years (3.0–8.7). In the primary outcome analysis, a total of 10 regimens were used, including Exenatide 2.0mg, Loxenatide 100ug, Loxenatide 200ug, Dulaglutide 1.5mg, Semaglutide 0.5mg, Semaglutide 1.0mg, Dulaglutide 0.75mg, Dulaglutide 3.0mg, Dulaglutide 4.5mg, Semaglutide 2.0mg. All above are weekly preparations.
Table 1.
Basic information included in the study.
| First author (yr) | Country | Study design | Study period | Intervention | Population characteristics | Primary outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | Age (mean ± sd) | Weight (mean ± sd) | BMI (mean ± sd) | Baseline HbA1c (%) | Diabetes duration (yr) | SBP, mm Hg | DBP, mm Hg | Race, Asian/Others, n (%) | Male sex, n (%) | ||||||
| Gadde (2017) | USA | RCT | 28-wk | ExenatideQWS-AI | 181 | 53.4 ± 9.8 | 89.2 ± 21.4 | 32.1 ± 5.4 | 8.4 ± 1.0 | 8.5 ± 6.3 | 127.6 ± 12. 8 | 77.4 ± 8.9 | 9 (5.0) | 89 (49.2) | HbA1c% |
| Placebo | 61 | 53.4 ± 9.5 | 89.0 ± 20.1 | 31.5 ± 5.1 | 8.5 ± 1.0 | 8.7 ± 5.8 | 127.7 ± 15.5 | 77.0 ± 8.1 | 3 (4.9) | 37 (60.7) | |||||
| Chen (2016) | China | RCT | 12 wk | PEX168100ug | 41 | 52.6 ± 8.4 | NA | 27.2 ± 3.6 | 8.23 ± 0.88 | 4.12 ± 6.42 | 128 ± 11 | 78.1 ± 9.4 | NA | 22 (53.66%) | HbA1c% |
| PEX168200ug | 39 | 49.8 ± 10.9 | NA | 26.3 ± 3.3 | 8.34 ± 1.15 | 4 ± 5.67 | 126 ± 12 | 78.1 ± 8.0 | NA | 22 (56.41%) | |||||
| Placebo | 38 | 53.5 ± 10.2 | NA | 27.2 ± 4.5 | 8.28 ± 1.05 | 6.46 ± 7.92 | 130 ± 14 | 77.8 ± 9.0 | NA | 26 (68.42%) | |||||
| Gao (2020) | China | RCT | 24 wk | PEX168100ug | 179 | 53.6 ± 10.5 | 71.2 ± 12.8 | 26.0 ± 3.5 | 8.5 ± 0.9 | 4.3 ± 3.5 | 124.3 ± 11.9 | 77.1 ± 8.0 | NA | 102 (57.0) | HbA1c% |
| PEX168200ug | 175 | 52.8 ± 10.6 | 73.6 ± 14.6 | 26.6 ± 3.8 | 8.5 ± 0.9 | 4.8 ± 3.2 | 125.5 ± 12.1 | 78.5 ± 8.2 | NA | 106 (60.6) | |||||
| Placebo | 179 | 52.3 ± 10.7 | 73.8 ± 14.1 | 26.6 ± 3.8 | 8.6 ± 0.9 | 4.7 ± 3.4 | 126.8 ± 11.5 | 79.3 ± 8.0 | NA | 98 (54.7) | |||||
| Ji (2020) | China | RCT | 30 wk | Sema0.5mg | 288 | 53.0 ± 11.4 | 77.6 ± 16.4 | 28.2 ± 5.0 | 8.1 ± 0.9 | 6.3 ± 5.4 | 128.8 ± 14.6 | 80.3 ± 9.3 | 243 (84.4) | 160 (55.6) | HbA1c% |
| Sema1.0mg | 290 | 53.0 ± 10.6 | 76.1 ± 16.3 | 27.9 ± 5.0 | 8.1 ± 0.9 | 6.7 ± 4.9 | 251 (86.6) | 154 (53.1) | |||||||
| Kim (2007) | USA | RCT | 15 wk | Exe2.0mg | 15 | 51 ± 11 | 110 ± 17 | 36 ± 6 | 8.3 ± 1.1 | 4 ± 5 | NA | NA | NA | 67% | HbA1c% |
| Placebo | 14 | 55 ± 9 | 101 ± 20 | 36 ± 6 | 8.6 ± 1.4 | 4 ± 4 | NA | NA | NA | 36% | |||||
| Pratley (2018) | USA | RCT | 40 wk | Sema0.5mg | 301 | 56 ± 10·9 | 96.4 ± 24.4 | 33.7 ± 7.1 | 8.3 ± 0.9 | 7.7 ± 5.9 | 134 ± 14.8 | 81 ± 9.0 | 50 (17) | 169 (56%) | HbA1c% |
| Dula0.75mg | 299 | 55 ± 10·4 | 95.6 ± 23.0 | 33.6 ± 6.9 | 8.2 ± 0.9 | 7.0 ± 5.5 | 133 ± 14.0 | 81 ± 8.9 | 48 (16) | 160 (54%) | |||||
| Sema1.0mg | 300 | 55 ± 10·6 | 95.5 ± 20.9 | 33.6 ± 6.5 | 8.2 ± 0.9 | 7.3 ± 5.7 | 133 ± 14.5 | 82 ± 9.1 | 38 (13) | 162 (54%) | |||||
| Dula1.5mg | 299 | 56 ± 10·6 | 93.4 ± 21.8 | 33.1 ± 6.6 | 8.2 ± 0.9 | 7.6 ± 5.6 | 132 ± 13.6 | 80 ± 8.7 | 55 (18) | 171 (57%) | |||||
| Grunberger (2012) | USA | RCT | 12 wk | Dula 1.5mg | 29 | 57.5 ± 7.9 | 85.8 ± 18.6 | 31.0 ± 4.3 | 7.3 ± 0.4 | 4.6 ± 4.1 | 127.3 ± 14.4 | 76.8 ± 9.2 | 14 (48.2) | 13 (44.8) | HbA1c(%) |
| Placebo | 32 | 55.0 ± 9.3 | 90.9 ± 18.9 | 32.1 ± 5.2 | 7.4 ± 0.6 | 3.9 ± 4.7 | 128.5 ± 12.3 | 77.9 ± 10.5 | 16 (50.0) | 18 (56.3) | |||||
| Umpierrez (2014) | USA | RCT | 26 wk | Dula 0.75mg | 270 | 56 ± 11 | 92 ± 19 | 33 ± 6 | 7.6 ± 0.9 | 3 ± 2 | 130 ± 16 | 80 ± 10 | 20 (7.4) | 118 (44) | HbA1c(%) |
| Dula 1.5mg | 269 | 56 ± 10 | 93 ± 19 | 34 ± 6 | 7.6 ± 0.9 | 3 ± 2 | 130 ± 16 | 79 ± 9 | 21 (7.8) | 114 (42) | |||||
| 52 wk | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||
| Davies (2017) | UK | RCT | 26 wk | Sema 1.0mg | 69 | 56.8 ± 11.8 | 88.8 ± 15.4 | 30.7 ± 4.0 | 7.8 ± 0.7 | 5.6 ± 5.0 | 134.2 ± 14.5 | 80.9 ± 8.9 | 10 (14.5) | 48 (69.6) | HbA1c(%) |
| Placebo | 71 | 58.9 ± 10.9 | 93.8 ± 18.1 | 32.6 ± 4.5 | 8.0 ± 0.8 | 6.7 ± 5.1 | 135.4 ± 15.5 | 80.6 ± 8.4 | 7 (9.9) | 40 (56.3) | |||||
| Eli Lilly and Company (2019) | USA | RCT | 18 wk | Dula 1.5mg | 81 | 57.65 ± 9.79 | NA | NA | 8.02 ± 0.8 | NA | NA | NA | 0/(0.00) | 39 (48.1) | HbA1c(%) |
| Dula 3.0mg | 79 | 55.90 ± 10.74 | NA | NA | 8.16 ± 0.92 | NA | NA | NA | 1 (1.3) | 35 (44.3) | |||||
| Dula 4.5mg | 76 | 57.13 ± 9.63 | NA | NA | 8.12 ± 0.81 | NA | NA | NA | 3 (3.9) | 36 (47.4) | |||||
| Placebo | 81 | 56.52 ± 8.93 | NA | NA | 8.08 ± 0.79 | NA | NA | NA | 0 (0.00) | 48 (59.3) | |||||
| Eli Lilly and Company (2020) | USA | RCT | 36 wk | Dula 1.5mg | 612 | 57.8 ± 9.7 | NA | NA | 8.64 ± 0.94 | NA | NA | NA | 13 (2.1) | 298 (48.7) | HbA1c(%) |
| Dula 3.0mg | 616 | 56.9 ± 10.2 | NA | NA | 8.63 ± 1.00 | NA | NA | NA | 18 (2.9) | 328 (53.2) | |||||
| Dula 4.5mg | 614 | 56.6 ± 10.2 | NA | NA | 8.64 ± 0.91 | NA | NA | NA | 14 (2.3) | 318 (51.8) | |||||
| Juan (2021) | USA | RCT | 40 wk | Sema 1.0mg | 481 | 58.2 ± 9.9 | NA | NA | NA | NA | NA | NA | 36 (7.5) | 284 (59) | HbA1c(%) |
| Sema 2.0mg | 480 | 57.9 ± 10.0 | NA | NA | NA | NA | NA | NA | 33 (6.9) | 279 (58.1) | |||||
HbA1c = glycosylated hemoglobin type A1C, RCTs = randomized clinical trials.
3.2. Quality assessment
We evaluated the risk of bias of the included studies using Cochrane Risk of Bias Tools. We found that 10 of these studies had a low risk of bias. The lack of participant blinding in the other 2 studies meant that they were open label RCTs. Participants dropped out in each study and were lost to follow-up in 8. None of these 8 studies reported resolution of missing data. Based on these limitations, the quality of evidence for our assessment of joint effects was reduced. For the remaining biases, most studies were moderate or low risk, and details are shown in (See Figure S1a, Supplemental Digital Content, http://links.lww.com/MD/J193, S1b, Supplemental Digital Content, http://links.lww.com/MD/J194, which illustrates the risk-of-bias graph/summary).
3.3. Efficacy outcomes
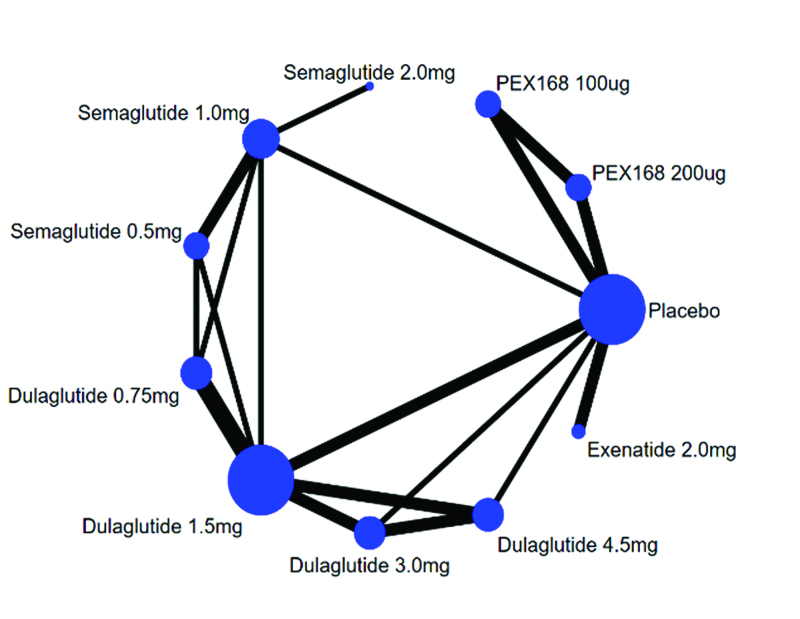
The network evidence of eligible comparisons for efficacy (ΔHbA1c, Δ FBG, Δ weight) were showed in Figure 2. All 12 trials considered in the NMA formed a connected network that compared placebo to 10 interventions. The agents were injected subcutaneously. All included studies used changes in HbA1c as the primary end point.
Figure 2.
Network of treatment comparisons for network meta-analysis. Each circular node represents a type of treatment. The node size is proportional to the total number of patients receiving a treatment. Each line represents a type of head-to-head comparison. The width of lines is proportional to the number of trials comparing the connected treatments.
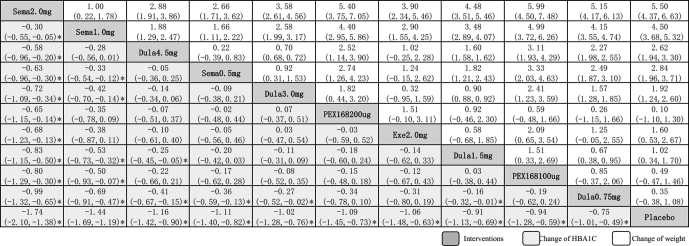
All GLP-1RAs significantly decreased HbA1c compared with placebo, and Semaglutide 2.0mg had the best hypoglycemic effect, with SMD between of −0.74% (95% CI: −2.10%, −1.38%) with significant different. Followed by Semaglutide 1.0mg [−1.44% (95% CI: −1.69%, −1.19%)], Dulaglutide 4.5mg [−1.16% (95% CI: −1.42%, −0.90%)], Semaglutide 0.5mg [−1.11 (95% CI: −1.40, −0.82)], Dulaglutide 3.0mg [−1.02 (95% CI: −1.28, −0.76)], PEX168 200ug [−1.09 (95% CI: −1.45, −0.73)], Exenatide 2.0mg [−1.06 (95% CI: −1.48, −0.63)], Dulaglutide 1.5mg [−0.91 (95% CI: −1.13, −0.69)], PEX168 100ug [−0.94 (95% CI: −1.28, −0.59)] and Dulaglutide 0.75mg [−0.75 (95% CI: −1.01,−0.49)]. Significant different could also be found in Semaglutide 2.0mg versus all the other interventions, which all prove that Semaglutide 2.0mg get the best hypoglycemic effect (Fig. 3).
Figure 3.
Lague of inclined interventions in evaluating change of HBA1C (A) and weight (B). Data in each cell are hazard or odds ratios (95% credible intervals) for the comparison of row-defining treatment versus column-defining treatment. Significant results are marked*. Dula = dulaglutide, Exe = exenatide, HbA1c = glycosylated hemoglobin type A1C, Sema = Semaglutide.
Different from HbA1c, in terms of weight loss, other GLP-1RAs had better weight loss effect except PEX168 100ug [0.49 kg (95% CI: −0.47, 1.46 kg)] was not as effective as placebo. Among them, Semaglutide 2.0mg had the best weight loss effect, with SMD between of −5.51 kg (95% CI: −6.64, −4.37 kg). Followed by Dulaglutide 0.75mg [−4.51 kg (95% CI: −5.33, −3.68 kg)], Semaglutide 1.0mg [−2.84 kg (95% CI: −3.72, −1.97 kg)], Dulaglutide 4.5mg [−2.62 kg (95% CI: −3.31, −1.94 kg)], Dulaglutide 3.0mg [−1.92 kg (95% CI: −2.61, −1.24 kg)], Exenatide 2.0mg [−1.60 kg (95% CI: −2.67, −0.53 kg)], Semaglutide 0.5 mg [−1.02 kg (95% CI: −1.71, −0.34 kg)], Dulaglutide 1.5 mg [−0.36 kg (95% CI: −1.09, −0.37 kg)] and PEX168 200ug [−0.10 kg (95% CI: −1.30, 1.10 kg)]. There was also a significant difference with Semaglutide 2.0 mg compared to all other interventions, all demonstrating that Semaglutide 2.0 mg also had a favorable effect on weight loss (Fig. 3).
The results of lowering fasting blood glucose were about the same as the results of lowering HbA1c. All GLP-1RAs significantly reduced HbA1c, and Semaglutide 2.0 mg had the best hypoglycemic effect with SMD between −2.71% (95% CI: −3.46%, −1.96%). The main difference was that Exenatide 2.0mg [−2.07% (95% CI: −2.93%, −1.22%)] was more effective in lowering fasting blood glucose, followed by Semaglutide 2.0mg and Semaglutide 1.0mg [−2.51% (95%) CI: −3.00%, −2.02%)]; the efficacy of other GLP-1RAs in reducing fasting blood glucose was similar. Dulaglutide 4.5mg [−1.98% (95% CI: −2.58%, −1.38%)], Semaglutide 0.5mg [−1.87 (−2.43, −1.30)], Dulaglutide 3.0mg [−1.75 (−2.33, −1.16)], Dulaglutide 1.5mg [−1.71 (−2.18, −1.24)], Dulaglutide 0.75mg [−1.45 (−1.98, −0.91)], PEX168 200ug [−1.16 (−1.76, −0.56)] and PEX168 100ug [−1.07 (−1.67, −0.46)] (See Figure S2, Supplemental Digital Content, http://links.lww.com/MD/J195, Supplemental Content, which illustrates the results of NMA of the clinical efficacy of ten GLP-1RAs).
3.4. Safety outcomes
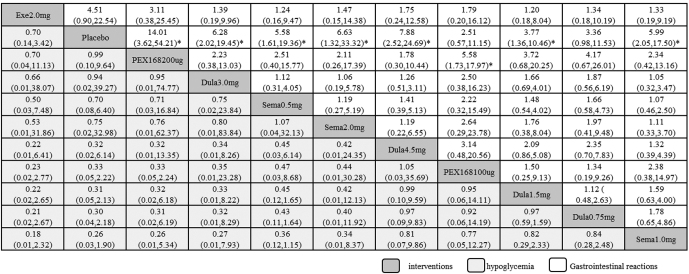
Results of safety and clinical efficacy were inconsistent, and serious AE, such as severe hypoglycemia, were rare among all GLP-1RAs. However, common adverse reactions such as gastrointestinal reactions and hypoglycemia often occur. Compared with placebo, the incidence of hypoglycemia was lower for all weekly formulations of GLP-1RAs except Exenatide 2.0 mg [0.70 (95% CI: 0.14–3.42)]. The lowest incidence was Semaglutide 1.0mg, OR was 3.91 (95% CI: 0.53–29.03), followed by Dulaglutide 0.75mg [3.28 (95% CI: 0.46–23.39)], Dulaglutide 1.5mg [3.19 (95% CI: 0.47–21.68)], Dulaglutide 4.5mg [3.17 (95% CI: 0.16–61.67)], PEX168 100ug [3.02 (95% CI: 0.45–20.25)], Semaglutide 0.5mg [1.42 (95% CI: 0.16–12.92)], Semaglutide 2.0mg[1.33 (95% CI: 0.03–58.15)], Dulaglutide 3.0mg [1.06 (95% CI: 0.03–44.49)], PEX168 200ug [1.01 (95% CI: 0.10–9.77)] and Exenatide 2.0mg [0.70 (95% CI: 0.14–3.42)] (Fig. 4).
Figure 4.
Comparisons for the safety of the 10 interventions. Data in each cell are hazard or odds ratios (95% credible intervals) for the comparison of row-defining treatment versus column-defining treatment. Significant results are marked*. Dula = dulaglutide, Exe = exenatide, Sema = Semaglutide.
Incidence of Gastrointestinal Reactions such as Nausea: The incidence of all GLP-1RAs was lower than placebo. The least nausea occurred was PEX168 200ug, with OR value of 14.01 (95% CI: 3.62–54.21), followed by Dulaglutide 4.5mg [7.88 (95% CI: 2.52–24.69)], Semaglutide 2.0mg [6.63 (95% CI: 1.32–33.32)]. Dulaglutide 3.0mg [6.28 (95% CI: 2.02–19.45)]. Semaglutide 1.0mg [5.99 (95% CI: 2.05–17.50)]. Semaglutide 0.5mg [5.58 (95% CI: 1.61–19.36)]. Exenatide 2.0mg [4.51 (95% CI: 0.90–22.54)]. Dulaglutide 1.5mg [3.77 (95% CI: 1.36–10.46)]. Dulaglutide 0.75mg [3.36 (95% CI: 0.98–11.53)]and PEX168 100ug [2.51 (95% CI: 0.57–11.15)] (Fig. 4).
Overall, semaglutide 2.0 mg has good efficacy and a favorable safety profile, and we recommend semaglutide as a treatment for patients with T2DM.
4. Discussion
This NMA included 12 studies covering 6213 individuals and primarily compared the HbA1c lowering effects of 4 GLP-1RA regimens with placebo and with each other. As expected, most GLP-1RA regimens significantly reduced HbA1c in the base case. However, the HbA1c-lowering effects of different regimens varied, with Semaglutide 2.0mg QW, Semaglutide 1.0mg QW, Dulaglutide 4.5mg QW, Semaglutide 0.5mg QW, Dulaglutide 3.0mg QW, 200ug QW relatively superior in the spectrum one end. The effects on fasting blood sugar and weight loss varied, for example, PEX168 100ug was less effective than placebo in weight loss. The other GLP-1RAs had better effects on fasting blood glucose and body weight than placebo. In terms of safety, the frequency of hypoglycemia in the 5 regimens of Semaglutide 1.0mg, Dulaglutide 0.75mg, Dulaglutide 1.5mg, PEX168 100ug, and Dulaglutide 4.5mg was significantly lower than the other 5 regimens and placebo. Therefore, it can be seen that although Semaglutide 2.0mg has the best efficacy, the incidence of AE of hypoglycemia is relatively higher than that of the above 5 regimens. Therefore, we need to strike a balance between efficacy and safety in clinical drug use, because no drug can guarantee absolute safety while having the best efficacy. To develop an individualized dosing regimen for patients can improve clinical efficacy and ensure drug safety.
GLP-1RAs as an emerging drug developed for the treatment of T2DM, not only achieves remarkable results in hypoglycemic therapy, but also helps diabetic patients lose weight. GLP-1RA works by reducing appetite and hunger, slowing the release of food from the stomach, increasing the feeling of fullness after eating. Since many people face difficulties maintaining weight loss, lifelong treatment may be required. In clinical trials, GLP-1RA was well tolerated and effective in helping people prevent weight regain, which may be a good option for long-term weight control and reducing the chance of serious health problems in patients.[18]
The “younger” trend of diabetes is becoming more and more obvious. The launch of GLP-1 weekly preparation brings convenience to patients.[3] The most common adverse reactions are hypoglycemia and gastrointestinal reactions such as nausea, vomiting and diarrhea, which are mostly transient and mainly occur at the beginning of treatment for a few weeks, and then gradually weakens or stabilizes over time. Based on this NMA, with the exception of an increased incidence of hypoglycemia in the Exenatide 2.0 mg group compared with placebo, other GLP-1RAs had lower incidences of both hypoglycemia and gastrointestinal reactions than placebo. It can be seen that most of the GLP-1RAs are relatively safe.
Semaglutide is approved in China in 2021. Previously, there was no specific analysis of GLP-1RA, which is approved weekly in China. Therefore, the indirect comparative evidence established through this study provides important clinical evidence for Chinese physicians who treat more than a quarter of the world diabetic patients.[19] In addition, the 2 regimens in this analysis, Semaglutide and Loxenatide, were not previously indirectly compared in the NMA of any GLP-1RAs, which may be related to the unique combination of GLP-1RAs in China.
As a newly marketed weekly preparation of GLP-1RAs, Semaglutide can significantly reduce the glycated hemoglobin of patients with T2DM regardless of the original treatment measures and the duration of the disease.[20,21] The greater the drop after the peptide, and the “smart” weight loss can be achieved while reducing blood sugar. In general, long-acting formulations reduced HbA1c more than short-acting formulations, with Semaglutide having the greatest HbA1c reduction.[22] And for Semaglutide, the 2.0mg weekly preparation has a more obvious hypoglycemic effect than the 1.0mg weekly preparation, but there is a lack of high-quality research evidence, and further research is needed. Although Semaglutide is the best among GLP-1RAs in terms of clinical efficacy, it is not very stable in terms of safety and still has a high incidence of gastrointestinal reactions.[23–25]
This meta-analysis is more comprehensive than previous studies and includes all marketed weekly GLP-1RAs formulations and different doses commonly used in clinical practice. We put more emphasis on the efficacy and differences between the 2 in clinical treatment, as well as the efficacy and adverse reactions of different doses. Global inconsistency is not evident in inconsistent model analysis. At the same time, the research bias was tested. As shown in the figure, the funnel plot is roughly symmetrical, indicating that the article has no obvious bias (See Figure S3, Supplemental Digital Content, http://links.lww.com/MD/J196, which illustrates Funnel plots).
This study has several potential limitations. First, basic information such as race and ethnicity were not extracted, and differences in the prevalence and etiology of T2DM between races and ethnicities may influence treatment response. Secondly, the treatment duration of the included studies varies greatly, ranging from 12 weeks to 52 weeks, and no in-depth research has been carried out on this, so the clinical efficacy of the specific treatment course of GLP-1RA needs to be further explored.
5. Conclusion
The 4 GLP-1RAs had better hypoglycemic and weight loss effects on T2D adults. Among them, Semaglutide 2.0mg has the best efficacy, including relatively low incidence of AE. Therefore, in terms of clinical medication, while ensuring the clinical efficacy of patients, we also need to pay more attention to the adverse reactions of drugs and monitor blood sugar at all times.
Acknowledgments
Thanks to the staff who helped with this study.
Author contributions
Conceptualization: Ying-Shi Zhang, Qing-Chun Zhao.
Data curation: Han Chen, Xin-Zhu Li, Jia-Qing Chen.
Formal analysis: Xin-Zhu Li, Jia-Qing Chen.
Investigation: Ying-Shi Zhang.
Methodology: Xin-Zhu Li, Tian-Shu Ren, Yi-Nuo Wang.
Project administration: Han Chen, Tian-Shu Ren, Qing-Chun Zhao.
Resources: Ying-Shi Zhang, Yi-Nuo Wang.
Supervision: Xin-Zhu Li.
Validation: Xin-Zhu Li.
Writing – original draft: Han Chen, Xin-Zhu Li, Jia-Qing Chen.
Writing – review & editing: Han Chen, Tian-Shu Ren, Ying-Shi Zhang, Qing-Chun Zhao.
Supplementary Material
Abbreviations:
- AE
- adverse events
- CI
- confidence interval
- GLP-1-RA
- glucagon-like peptide 1 receptor agonists
- HbA1c
- glycosylated hemoglobin type A1C
- NMA
- network meta-analysis
- ORs
- odd ratios
- RCTs
- randomized clinical trials
- SMD
- standardized mean differences
- T2DM
- diabetes mellitus type 2
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
Ethics: This study is a meta-analysis that does not involve ethical norms and there is no need for ethical approval.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Chen H, Li X-Z, Chen J-Q, Ren T-S, Zhang Y-S, Wang Y-N, Zhao Q-C. Comparative efficacy and safety of glucagon-like peptide 1 receptor agonists for the treatment of type 2 diabetes: A network meta-analysis. Medicine 2023;102:27(e34122).
Contributor Information
Han Chen, Email: chenjq0506@163.com.
Xin-Zhu Li, Email: Liii_43095@163.com.
Jia-Qing Chen, Email: chenjq0506@163.com.
Tian-Shu Ren, Email: sz_pharm@163.com.
Ying-Shi Zhang, Email: zhangyingshi526@163.com.
Yi-Nuo Wang, Email: 492531917@qq.com.
References
- [1].Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. [DOI] [PubMed] [Google Scholar]
- [2].Andreasen CR, Andersen A, Knop FK, et al. Understanding the place for GLP-1RA therapy: translating guidelines for treatment of type 2 diabetes into everyday clinical practice and patient selection. Diabetes Obes Metab. 2021;23(Suppl 3):40–52. [DOI] [PubMed] [Google Scholar]
- [3].Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [6].Gadde KM, Vetter ML, Iqbal N, et al. Efficacy and safety of autoinjected exenatide once-weekly suspension versus sitagliptin or placebo with metformin in patients with type 2 diabetes: the DURATION-NEO-2 randomized clinical study. Diabetes Obesi Metab. 2017;19:979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen X, Lv X, Yang G, et al. Polyethylene glycol loxenatide injections added to metformin effectively improve glycemic control and exhibit favorable safety in type 2 diabetic patients. Journal of Diabetes. 2016;9:158–67. [DOI] [PubMed] [Google Scholar]
- [8].Gao F, Lv XF, Mo ZH, et al. Efficacy and safety of polyethylene glycol loxenatide as add-on to metformin in patients with type 2 diabetes: a multicentre, randomized, double-blind, placebo-controlled, phase 3b trial. Diabetes Obesi Metab. 2020;22:2375–83. [DOI] [PubMed] [Google Scholar]
- [9].Ji LN, Dong XL, Eliaschewitz F, et al. Efficacy and safety of once-weekly semaglutide vs. once-daily sitagliptin as add-on to metformin in subjects with Type 2 Diabetes (SUSTAIN China MRCT): a 30-week double-blind, Phase 3a, Randomised Trial. Diabetes, Obesity and Metabolism. 2020;23:404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leahy JL. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with Type 2 Diabetes. Diabetes Care. 2007;30:1487–93. [DOI] [PubMed] [Google Scholar]
- [11].Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. The Lancet Diabetes & Endocrinology. 2018;6:275–86. [DOI] [PubMed] [Google Scholar]
- [12].Grunberger G, Chang A, Soria GG, et al. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with Type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabet Med. 2012;29:1260–7. [DOI] [PubMed] [Google Scholar]
- [13].Umpierrez G, Tofé Povedano S, Pérez Manghi F, et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37:2168–76. [DOI] [PubMed] [Google Scholar]
- [14].Davies M, Pieber TR, Hartoft-Nielsen ML, et al. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with Type 2 diabetes: a randomized clinical trial. JAMA. 2017;318:1460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eli L. Company. A study of the efficacy and safety of dulaglutide (LY2189265) in participants with type 2 diabetes - Full Text View - ClinicalTrials.gov..
- [16].Nct. A study of investigational dulaglutide doses in participants with type 2 diabetes on metformin monotherapy - Full Text View - ClinicalTrials.gov..
- [17].Jpf A, Pa B, Hsb C, et al. Efficacy and safety of once-weekly semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. The Lancet Diabetes & Endocrinol. 2021;9:563–74.. [DOI] [PubMed] [Google Scholar]
- [18].Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38:2821–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- [20].Andreadis P, Karagiannis T, Malandris K, et al. Semaglutide for type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:2255–63. [DOI] [PubMed] [Google Scholar]
- [21].Li J, He K, Ge J, et al. Efficacy and safety of the glucagon-like peptide-1 receptor agonist oral semaglutide in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;172:108656. [DOI] [PubMed] [Google Scholar]
- [22].Meier JJ. Efficacy of semaglutide in a subcutaneous and an oral formulation. Front Endocrinol (Lausanne). 2021;12:645617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gallwitz B, Giorgino F. Clinical perspectives on the use of subcutaneous and oral formulations of semaglutide. Front Endocrinol (Lausanne). 2021;12:645507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smits MM, Van Raalte DH. Safety of semaglutide. Front Endocrinol (Lausanne). 2021;12:645563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.