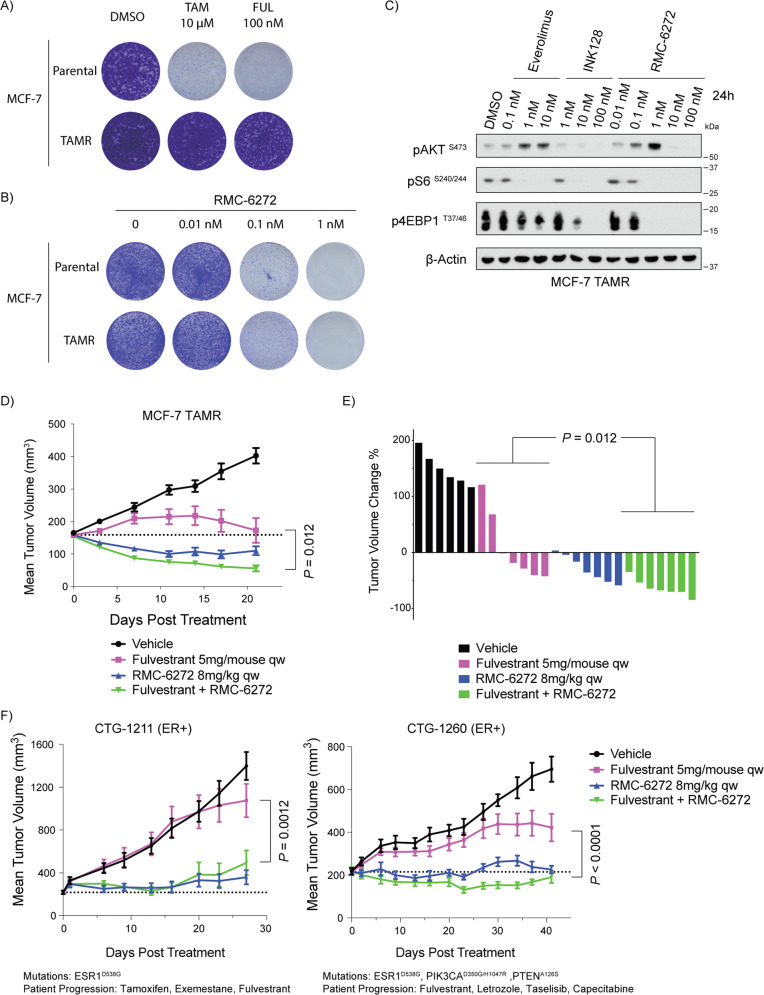
Fig. 5. RMC-6272 remains effective in both in vitro and in vivo hormone therapy-resistant breast cancer models.
A Crystal violet staining of parental or tamoxifen-resistant (TAMR) MCF-7 cells grown in the presence of 10 μM tamoxifen (TAM), 100 nM fulvestrant (FUL), or DMSO as a control. B Crystal violet staining of parental or tamoxifen-resistant (TAMR) MCF-7 cells grown in the presence of various concentrations of RMC-6272. C Immunoblot of lysates of MCF-7 TAMR cells treated with Everolimus, INK128 or RMC-6272 for 4 h at indicated doses. Image represents at least two independent experiments. D Mice carrying MCF-7 TAMR orthotopic xenograft tumors were treated with vehicle, fulvestrant, RMC-6272, or the combination for 21 days. n = 7 per group except for vehicle which was n = 6. E Relative change in tumor volume of individual mice at day 21 colored by treatment group. F Single and combination agent dosing in two patient-derived xenograft models derived from ER+ breast cancer patients experiencing acquired resistance to hormone therapies. n = 9 mice for each arm in CTG-1211 and n = 9 for each arm in CTG-1260 except for vehicle group in CTG-1260 which was n = 4 beginning day 9. PI3K pathway mutation and previous patient treatments are indicated. P values based on two-tailed Student’s t-test.