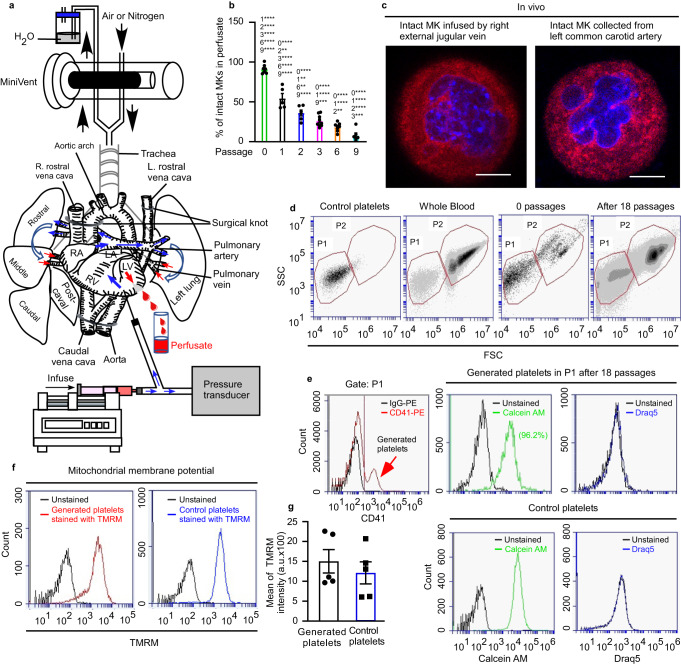
Fig. 1. Mouse platelets are generated from megakaryocytes passaged multiple times through mouse pulmonary vasculature ex vivo.
Mouse megakaryocytes (MKs), labelled with CD41-PE or CD41-FITC antibodies, were passaged repeatedly through the pulmonary vasculature ex vivo. Lungs were ventilated with air throughout (b, d–g). a Diagram illustrating the approach to generating mouse platelets. End-expiratory positive pressure was applied to prevent lung collapse. b Intact MKs from perfusates after passaging the indicated number of times through lung vasculature. Quantification was from ≥250 fields of view, counting ≥230 cells in total and displayed as a percentage of total number of cells. Numbers above each column indicate significant differences to other passages. N = 7, 5, 6, 9, 8 and 6 independent experiments for passage numbers 0, 1, 2, 3, 6 & 9. Error bars are mean ± SEM. Two-way ANOVA with Tukey’s multiple comparisons test, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. c In vivo demonstration that intact mouse MKs pass through the pulmonary vasculature. Mouse MKs were stained with CellTracker™ Red CMTPX dye (red) and Hoechst 33342 (blue) prior to injection into the right external jugular vein of an anaesthetized recipient C57BL/6 mouse. Blood was collected from the left common carotid artery and cells were imaged by confocal fluorescence microscopy. Images shown are representative of n = 4 independent experiments. Scale bar: 10 µm. d Gating strategy for quantification of generated platelets. The number of generated platelets in the perfusate collected after the 18th passage was determined by the number of CD41(+) events in gate P1. e Events in P1 gate (from the experiment shown in Fig. 1d) are defined as generated platelets (indicated by the red arrow), with higher mean fluorescence compared to those derived from control IgG-PE- treated MKs. Gate P1 also captures CD41-negative cells, which include stem cells and host-derived platelets. Viability of generated platelets, and whether they contain DNA, were checked by Calcein-AM and DRAQ5 dyes, respectively. f Mitochondrial membrane potential in generated and control platelets was determined by Tetramethyl rhodamine methyl ester (TMRM) accumulation in active mitochondria and measured by FACS. g TMRM signals from (f) were quantified and displayed as mean ± SEM. N = 5 independent experiments; two-tailed unpaired t test. Source data are provided in the Source Data file.