Summary
Background
Several psychiatric disorders have been associated with increased risk of cardiovascular disease (CVD), however, the role of familial factors and the main disease trajectories remain unknown.
Methods
In this longitudinal cohort study, we identified a cohort of 900,240 patients newly diagnosed with psychiatric disorders during January 1, 1987 and December 31, 2016, their 1,002,888 unaffected full siblings, and 1:10 age- and sex-matched reference population from nationwide medical records in Sweden, who had no prior diagnosis of CVD at enrolment. We used flexible parametric models to determine the time-varying association between first-onset psychiatric disorders and incident CVD and CVD death, comparing rates of CVD among patients with psychiatric disorders to the rates of unaffected siblings and matched reference population. We also used disease trajectory analysis to identify main disease trajectories linking psychiatric disorders to CVD. Identified associations and disease trajectories of the Swedish cohort were validated in a similar cohort from nationwide medical records in Denmark (N = 875,634 patients, same criteria during January 1, 1969 and December 31, 2016) and in Estonian cohorts from the Estonian Biobank (N = 30,656 patients, same criteria during January 1, 2006 and December 31, 2020), respectively.
Findings
During up to 30 years of follow-up of the Swedish cohort, the crude incidence rate of CVD was 9.7, 7.4 and 7.0 per 1000 person-years among patients with psychiatric disorders, their unaffected siblings, and the matched reference population. Compared with their siblings, patients with psychiatric disorders experienced higher rates of CVD during the first year after diagnosis (hazard ratio [HR], 1.88; 95% confidence interval [CI], 1.79–1.98) and thereafter (1.37; 95% CI, 1.34–1.39). Similar rate increases were noted when comparing with the matched reference population. These results were replicated in the Danish cohort. We identified several disease trajectories linking psychiatric disorders to CVD in the Swedish cohort, with or without mediating medical conditions, including a direct link between psychiatric disorders and hypertensive disorder, ischemic heart disease, venous thromboembolism, angina pectoris, and stroke. These trajectories were validated in the Estonian Biobank cohort.
Interpretation
Independent of familial factors, patients with psychiatric disorders are at an elevated risk of subsequent CVD, particularly during first year after diagnosis. Increased surveillance and treatment of CVDs and CVD risk factors should be considered as an integral part of clinical management, in order to reduce risk of CVD among patients with psychiatric disorders.
Funding
This research was supported by EU Horizon 2020 Research and Innovation Action Grant, European Research Council Consolidator grant, Icelandic Research fund, Swedish Research Council, US NIMH, the Outstanding Clinical Discipline Project of Shanghai Pudong, the Fundamental Research Funds for the Central Universities, and the European Union through the European Regional Development Fund; the Research Council of Norway; the South-East Regional Health Authority, the Stiftelsen Kristian Gerhard Jebsen, and the EEA-RO-NO-2018-0535.
Keywords: Psychiatric disorders, Cardiovascular disease, Sibling, Family design, Disease trajectory
Research in context.
Evidence before this study
We searched PubMed for studies on the association between various clinically diagnosed psychiatric disorders and subsequent cardiovascular disease of any type, from database inception to November 15, 2022, using search terms “psychiatric/mental”, “cardiovascular”, and “cohort/prospective/longitudinal”, with restrictions on journal articles. We summarized the evidence from these 47 studies (in Supplementary Material) which mostly reported a positive association between depression, anxiety and stress-related disorders, and hypertension, coronary heart disease and stroke. These studies were often limited to selected populations (conscripts), with self-reported data and incomplete follow-up. Importantly, the role of unmeasured confounding factors on the observed associations between diagnosed psychiatric disorders and cardiovascular disease, in particular familial factors (i.e., genetic susceptibility and early-life environmental factors shared within families), were not firmly addressed in these studies. No study has so far assessed the disease trajectories linking common psychiatric disorders and cardiovascular diseases, limiting our understanding of pathophysiological mechanisms whether the link between the two disease groups was direct or mediated through other medical conditions (i.e., indirect link).
Added value of this study
With nationwide complete information on family links in Sweden and Denmark, our study is the first to comprehensively investigate the role of familial factors in the association between psychiatric disorders and subsequent risk of incident cardiovascular disease through a sibling comparison. We demonstrated that independent of familial background, patients with psychiatric disorders had an increased risk of cardiovascular disease, with risk increment upmost during first year after psychiatric diagnosis and remained elevated up to thirty years of follow-up. We further identified several disease trajectories linking psychiatric disorders to cardiovascular disease in the Swedish cohort, with and without mediating medical conditions. The direct link between psychiatric disorders and hypertensive disorder, ischemic heart disease, venous thromboembolism, angina pectoris, and stroke, were independently validated in Estonian Biobank.
Implications of all the available evidence
Despite of the increasing knowledge on genetic correlations between several psychiatric disorders and cardiovascular diseases, our study provides solid evidence on the association between a wide range of psychiatric disorders and risk of incident cardiovascular disease and fatal cardiovascular events independent on shared familial factors, in Sweden and Denmark. The highest association noted during the first year after psychiatric diagnosis supports for an increased surveillance of cardiovascular comorbidities among newly diagnosed patients receiving a psychiatric diagnosis and highlight a critical time window for clinical management. The findings on several disease trajectories between psychiatric disorders and a list of cardiovascular diseases in Sweden and Estonian Biobank, contributing to a better understanding of the mechanisms underlying the development of cardiovascular disease following psychiatric disorders.
Introduction
Patients with psychiatric disorders are at higher risk of various medical conditions1 and premature death.2 The latter has predominantly been attributed to cardiovascular disease (CVD).3,4 We summarized 47 longitudinal cohort studies that investigated the association of various clinically diagnosed psychiatric disorders with selected CVDs (Appendix Table S1), mostly reporting positive associations. In addition to the often selected, healthy study populations (male or female conscripts),5, 6, 7, 8 self-reported data,9,10 and incomplete follow-up,5,6,11,12 the role of familial confounding was rarely addressed in the existing studies, except for two studies focusing on patients with anxiety or stress-related disorders.13,14 Considering the genetic correlation between psychiatric disorders and CVD15 as well as the importance of early life environment to the development of these two complex disease groups,16,17 it is plausible that the comorbidity between the two groups of diseases is partially attributed to unmeasured genetic and environmental factors shared within families (i.e., familial confounding).18 Thus, further studies are needed to assess the association between psychiatric disorders and CVD after accounting for familial confounding.
Further, the complex mechanisms and disease trajectories through which CVD develops in patients with psychiatric disorders has received little research attention.19 Exploration of disease trajectories may help improve our understanding of the underlying mechanisms. Disease trajectory analysis has recently been established as an approach to explore disease progression.20,21 One example is our recent study investigating the trajectories of medical conditions after a diagnosis of depression using disease network analysis, revealing cardiometabolic diseases, chronic inflammatory diseases, and diseases related to tobacco abuse, as main disease clusters following a diagnosis of depression.22 Some disease clusters were directly linked to depression (i.e., a direct disease pathway) whereas other subsequent diseases were mediated through other medical conditions (i.e., an indirect disease pathway). To the best of our knowledge, the disease trajectories linking other common psychiatric disorders and CVD remain to be explored.
Leveraging the nationwide registers in Sweden and Denmark with the possiblities for family-based comparisons and controling for multiple covariates and comorbid conditions, we aimed to investigate the role of familial factors in the association between all psychiatric disorders and subsequent risk of incident CVD. In order to improve our understanding of the directionality and disease progression, we further aimed to identify direct as well as indirect disease trajectories linking psychiatric disorders to CVD, exploring all common medical conditions following a diagnosis of psychiatric disorder in Sweden and validated the identified trajectories in the Estonian Biobank.
Methods
Study design
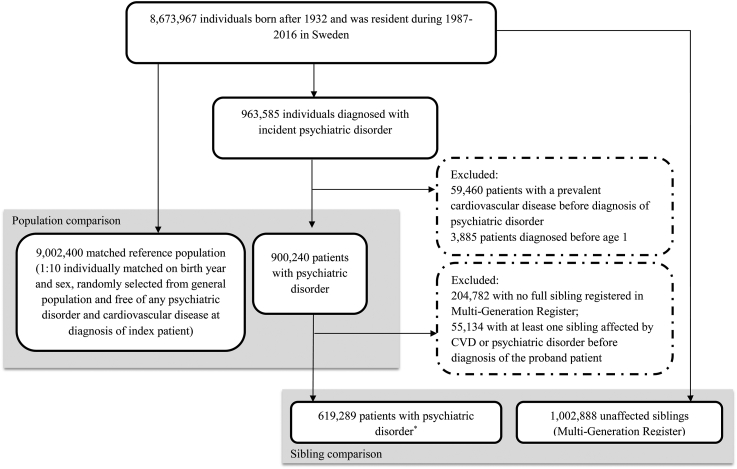
Using personal identification numbers uniquely assigned to all Swedish residents, we identified 963,585 individuals born after 1932 in Sweden who received a first diagnosis of any psychiatric disorder between 1 January 1987 and 31 December 2016.23 Patients diagnosed with psychiatric disorders under age 1 (N = 3885, possible congenital/developmental anomalies) or with a prevalent CVD before diagnosis of psychiatric disorders (N = 59,460) were excluded, leaving 900,240 patients with a newly diagnosed psychiatric disorder in the analysis (Fig. 1). Date of first diagnosis of psychiatric disorder was used as the index date for the exposed patients.
Fig. 1.
Flowchart of study design and study participants for Swedish cohort. ∗57,779 families had more than one sibling with psychiatric disorders.
The Swedish Multi-Generation Register includes largely complete familial linkage with information on mothers for 97% and on fathers for 95% of individuals born since 1932 and alive on January 1st 1961.24 Through the Register, we identified 1,002,888 unaffected full siblings for 619,289 patients with psychiatric disorders (68.8% of all exposed patients) who were alive and free of psychiatric disorders and CVD before diagnosis of the index patient. For each exposed patient, we randomly selected 10 age- and sex-matched individuals from the study base using the method of incidence density sampling (N = 9,002,400).25 This method aims to select a control group that mimics closely the underlying source population from which the exposed individuals arise. The date of diagnosis for the index patient was used as the index date for the unaffected siblings and matched reference population.
We followed all study participants from the index date until first diagnosis of any CVD, death, emigration, first diagnosis of psychiatric disorders (for unaffected siblings and matched reference population), or the end of the study period (31 December 2016), whichever occurred first.
The study was approved by the Ethical Vetting Board in Stockholm, Sweden (DNRs 2012/1814-31/4 and 2015/1062-32), the Danish Health Data Authority (project no. FSEID-00003339) and the Estonian Committee on Bioethics and Human Research (24 March 2020, nr 1.1-12/624).
Ascertainment of psychiatric disorders and cardiovascular disease
We used the 9th and 10th Swedish revisions of the International Classification of Diseases (ICD-9 and 10) codes to identify psychiatric disorders and CVD attended by specialized care (Appendix Table S2). From the Swedish Patient Register, we defined incident psychiatric disorders as any first inpatient or outpatient hospital visit with a psychiatric disorder as the primary diagnosis. CVD was ascertained as any first inpatient or outpatient visit with CVD as the primary diagnosis (from the Patient Register), or as a death with CVD as the underlying cause (from the Causes of Death Register). The diagnostic accuracy of CVD is generally considered reliable in the Patient Register. We also identified fatal cardiovascular event as any death within 30 days of incident CVD according to the Causes of Death Register.
We classified psychiatric disorders as non-affective psychotic disorders, affective psychotic disorders, substance misuse, non-psychotic mood disorder, anxiety, and stress-related disorders, eating disorder, personality disorders, attention deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and intellectual disability.26 Consistent with our previous work,14 we categorized CVD as ischemic heart disease, cerebrovascular disease, emboli and thrombosis, hypertensive disease, heart failure, and arrhythmia/conduction disorder.
Covariates
We obtained educational level, individualized family income, and cohabitation status from the Longitudinal Integration Database for Health Insurance and Labor Market.27 Participants with missingness on these variables were grouped as unknown or missing for categorical variables. Having any of the following conditions before the index date was defined as having a history of somatic diseases: chronic pulmonary disease, connective tissue disease, diabetes, renal diseases, liver disease, ulcer diseases, malignancies, and HIV infection/AIDS (Appendix Table S2). We considered a family history of CVD as a diagnosis of any CVD or death from CVD among biological parents or full siblings of the study participants before the index date according to the Swedish Patient Register and Causes of Death Register.
Statistical analysis
The statistical analysis includes two steps, namely association analysis and disease trajectory analysis. In each step, we designed an exploration phase in the Swedish cohort and a replication/validation phase in Denmark and Estonian Biobank, respectively (Appendix Figure S2).
Association analysis
To visualize the temporal patterns of the association between psychiatric disorders and CVD, we used flexible parametric models to estimate the time-varying hazard ratio of incident CVD following a diagnosis of psychiatric disorders,28 by comparing the hazard of CVD in patients with psychiatric disorders to the hazard in their unaffected full siblings and matched reference population. Because we observed a marked increase of CVD rate immediately following a diagnosis of psychiatric disorders, we separately estimated the association within one year and beyond one year after diagnosis. We estimated the averaged hazard ratio and 95% confidence intervals (CIs) during the first year of follow-up and beyond, from stratified Cox models, using time since the index date as the underlying time scale. The proportional hazard assumption was tested using Schoenfeld residuals and found to hold. HRs were estimated for any event of first-onset CVD and by categories of this first-onset CVD. Because patients with psychiatric disorders are frequently diagnosed with multiple psychiatric disorders, in the analysis by groups of psychiatric disorders, we identified all diagnoses of psychiatric disorders during follow-up and considered psychiatric disorders as a time-varying variable. Patients contributed person-time to different psychiatric disorders after the first diagnosis of the respective psychiatric disorder, if more than one diagnosis of psychiatric disorders were registered after cohort entry.
We performed subgroup analyses by sex, age at index date (by tertile distribution: ≤22, 23–41, or ≥42 years), age at follow-up (<35 or ≥35 years), calendar year at index date (1987–1996, 1997–2006, or 2007–2016), history of somatic diseases (no or yes), and family history of CVD (no or yes). In the sibling comparison, Cox models were stratified by family identifier and adjusted for sex, birth year, educational level, individualized family income, cohabitation status, and history of somatic diseases. In the population comparison, Cox models were stratified by the matching variables (birth year and sex) and adjusted for all abovementioned covariates plus family history of CVD.
Replication of the association analysis in a Danish cohort
To assess whether the associations identified in the Swedish cohort could be generalized to an external population, we applied the same approach and identified a Danish cohort including 875,634 patients with first-onset psychiatric disorders, their unaffected full siblings (N = 625,710, among 46.1% of the patients with psychiatric disorders), and up to 1:10 age- and sex-matched reference population (N = 8,756,340). Details on study design are available in Appendix Figure S1 and Section 1.
Disease trajectory analysis
To identify disease trajectories following a diagnosis of first-onset psychiatric disorder, we applied disease trajectory analysis to explore all medical conditions diagnosed subsequent to a diagnosis of psychiatric disorders.20,21 This analysis was performed among patients with psychiatric disorders diagnosed between 2001 and 2016, due to the availability of outpatient records from 2001 onward in the Swedish Patient Register.23 We used the 3-digit ICD-10 codes for medical condition identification and clustered conditions with clinical or biological similarities.21 To minimize concerns of reverse causality, we included only medical conditions diagnosed more than six months after the diagnosis of psychiatric disorders.
The disease trajectory analysis is exploratory in nature with the aim to better understand the link between psychiatric disorders and subsequent CVDs. It included three steps: 1) we identified all subsequent medical conditions associated with a diagnosis of psychiatric disorder in a phenome-wide association analysis (PheWAS), by comparing patients with psychiatric disorders to matched population controls; 2) we determined the temporal order of the following two diseases in the trajectory among patients with a psychiatric disorder. To explore trajectories leading to any CVD, either disease 1 or disease 2 was restricted to be CVD; and 3) we assessed the magnitude of the association between the two diseases within each pair using conditional logistic regression (details in Appendix Section 2). Because the disease trajectory analysis explored two medical conditions following psychiatric disorders, per trajectory (e.g., psychiatric disorder to disease 1 [D1] to disease 2 [D2]), we concluded on a direct link between psychiatric disorder and CVD if D1 was any CVD, and referred to an indirect link if D2 was any CVD. The disease trajectory analysis was performed for all groups of psychiatric disorders except for ASD, due to the lack of associations between ASD and CVD in the association analysis.
Validation of the disease trajectory analysis in Estonian Biobank (EstBB)
EstBB is a population-based biobank at the Institute of Genomics at the University of Tartu with participants representing 20% adult population in Estonia.29 To validate if the disease trajectories identified in the Swedish cohort were generalizable to an external population, we constructed a matched cohort in EstBB comprising 30,656 patients with psychiatric disorders and up to 1:10 age- and sex-matched reference population to examine the identified direct disease pathways between psychiatric disorders and subsequent CVD. Detailed description of the EstBB database, construction of the validation cohorts, and analytical methods are available in Appendix Section 3.
Analyses were performed in SAS 9.4 (SAS Institute), STATA 16 (StataCorp LP) and R (version 4.1.2). Informed consent was waived by law using register-based data for the Swedish and Danish participants, and was provided by each participant in the Estonian Biobank at enrollment. Our study is reported according to the Strengthening the Reporting of Observational studies in Epidemiology checklist (STROBE) reporting guideline.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. QS, FF and UV had full access to the Swedish data, DHM and AB had full access to the Danish data, and LBL, SK and LM had full access to the Estonian data in the study, and take responsibility for the integrity and the accuracy of the data in the respective cohort. The corresponding authors had final responsibility for the decision to submit for publication, upon the approval from all authors.
Results
Association analysis
In the Swedish cohort, the median age of patients with psychiatric disorders was 28 years (Table 1), varying from 12 years for ASD to 39 years for affective psychotic disorder (Appendix Table S3). Although similar, patients with psychiatric disorders of the sibling comparison were younger than those of the population comparison (26 vs. 28 years). The population was similar in the Danish cohort (Appendix Table S4).
Table 1.
Characteristics of patients with psychiatric disorders, their unaffected siblings, and matched reference population–a Swedish register-based cohort.
| Characteristics | Sibling comparison |
Population comparison |
||||
|---|---|---|---|---|---|---|
| Exposed patients (N = 619,289) | Unaffected full siblings (N = 1,002,888) | P value | Exposed patients (N = 900,240) | Matched reference population (N = 9,002,400) | P value | |
| Median age at index date in years (IQR) | 26.00 (18.00–42.00) | 28.00 (18.00–44.00) | <0.0001 | 28.00 (18.00–44.00) | 28.00 (18.00–44.00) | – |
| Median follow-up time in years (IQR) | 7.96 (3.78–12.85) | 7.88 (3.62–13.08) | <0.0001 | 7.79 (3.68–12.70) | 7.58 (3.53–12.67) | <0.0001 |
| Sex | ||||||
| Female | 320,594 (51.77) | 489,919 (48.85) | <0.0001 | 465,819 (51.74) | 4,658,190 (51.74) | – |
| Male | 298,695 (48.23) | 512,969 (51.15) | <0.0001 | 434,421 (48.26) | 4,344,210 (48.26) | – |
| Educational level | ||||||
| <9 years | 186,406 (30.10) | 271,251 (27.05) | <0.0001 | 278,718 (30.96) | 1,969,745 (21.88) | <0.0001 |
| 9–12 years | 267,590 (43.21) | 439,550 (43.83) | 389,953 (43.32) | 3,937,777 (43.74) | ||
| >12 years | 165,293 (26.69) | 292,087 (29.12) | 231,569 (25.72) | 3,094,878 (34.38) | ||
| Yearly individualized family income level | ||||||
| Top 20% | 100,837 (16.28) | 223,287 (22.26) | <0.0001 | 123,287 (13.69) | 1,856,114 (20.62) | <0.0001 |
| Middle | 400,273 (64.63) | 571,858 (57.02) | 588,792 (65.40) | 5,344,817 (59.37) | ||
| Lowest 20% | 117,872 (19.03) | 207,563 (20.70) | 187,093 (20.78) | 1,795,221 (19.94) | ||
| Unknown | 307 (0.05) | 180 (0.02) | 1068 (0.12) | 6248 (0.07) | ||
| Cohabitation status | ||||||
| Non-cohabitating | 508,676 (82.14) | 738,088 (73.60) | <0.0001 | 733,124 (81.44) | 6,639,874 (73.76) | <0.0001 |
| Cohabitating | 110,306 (17.81) | 264,620 (26.39) | 166,048 (18.44) | 2,356,278 (26.17) | ||
| Missing | 307 (0.05) | 180 (0.02) | 1068 (0.12) | 6248 (0.07) | ||
| History of somatic diseasesa | 29,426 (4.75) | 32,506 (3.24) | <0.0001 | 46,550 (5.17) | 271,386 (3.01) | <0.0001 |
| Family history of cardiovascular diseaseb | 227,751 (36.78) | 392,399 (39.13) | <0.0001 | 337,832 (37.53) | 3,266,253 (36.28) | <0.0001 |
| Type of psychiatric disorders | ||||||
| 1. Non-affective psychotic disorders | 16,765 (2.71) | – | 24,023 (2.67) | – | ||
| 2. Affective psychotic disorders | 14,449 (2.33) | – | 21,421 (2.38) | – | ||
| 3. Substance misuse | 107,464 (17.35) | – | 161,192 (17.91) | – | ||
| 4. Non-psychotic mood disorders | 121,439 (19.61) | – | 176,450 (19.60) | – | ||
| 5. Anxiety and stress related-disorders | 200,143 (32.32) | – | 291,644 (32.40) | – | ||
| 6. Eating disorders | 20,136 (3.25) | – | 25,665 (2.85) | – | ||
| 7. Personality disorders | 9049 (1.46) | – | 13,575 (1.51) | – | ||
| 8. Attention deficit hyperactivity disorder (ADHD) | 54,706 (8.83) | 79,039 (8.78) | ||||
| 9. Autism spectrum disorder (ASD) | 18,762 (3.03) | 26,186 (2.91) | ||||
| 10. Intellectual disability | 13,432 (2.17) | 19,018 (2.11) | ||||
| 11. Others | 42,944 (6.93) | – | 62,027 (6.89) | – | ||
| Number of psychiatric diagnoses | ||||||
| One | 378,433 (61.11) | – | 546,572 (60.71) | – | ||
| Two | 130,753 (21.11) | – | 191,590 (21.28) | – | ||
| Three or more | 110,103 (17.78) | – | 162,078 (18.01) | – | ||
IQR: Interquartile range.
P value for difference was calculated by t test for continuous variables, and by Chi-square test for categorical variables.
History of somatic diseases included chronic pulmonary disease, connective tissue disease, diabetes, renal diseases, liver diseases, ulcer diseases, and HIV infection/AIDS diagnosed before index date.
The difference between exposed patients and unaffected full siblings was due to different number of siblings per exposed patient. The family history of cardiovascular disease was constant within each family.
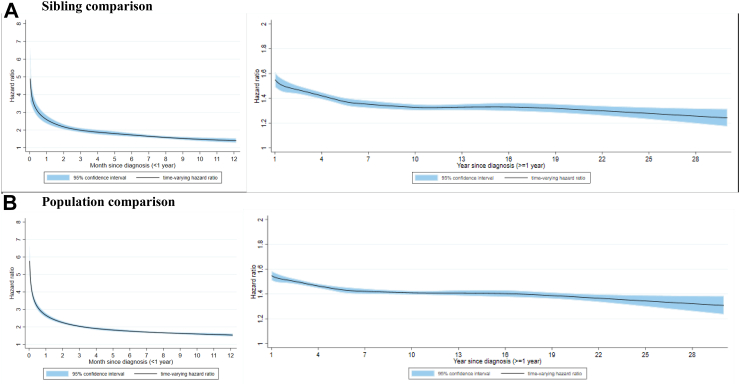
During up to 30 years of follow-up in the Swedish cohort, the crude incidence rate of any CVD was 9.7, 7.4, and 7.1 per 1000 person-years among the patients with psychiatric disorders, their unaffected full siblings, and matched reference population (Appendix Table S5). In the sibling comparison, we found a marked increase in risk of CVD shortly after a diagnosis of psychiatric disorders, which then declined gradually over time (Fig. 2): the HR was 1.88 (95% CI 1.79–1.98) within first year and 1.37 (95% CI 1.34–1.39) beyond first year after diagnosis (Appendix Table S6). Similar rate increases were noted when comparing to matched reference population (HR 1.91 [95% CI 1.86–1.96] within first year and 1.41 [95% CI 1.40–1.43] beyond first year since diagnosis). We observed similar associations by sex, age, calendar year, history of somatic diseases, or family history of CVD (Appendix Table S5).
Fig. 2.
Time-varying hazard ratio for an incident cardiovascular disease among patients with psychiatric disorders, compared to their unaffected full siblings (sibling comparison) or matched reference population (population comparison), by time since psychiatric diagnosis (<1 or ≥1 year)∗. A) Sibling comparison. B) Population comparison. ∗ Time-varying hazard ratios and 95% confidence intervals were derived from flexible parametric survival models, allowing the effect of psychiatric disorder to vary over time. A spline with 5 df was used for the baseline rate, and 3 df was used for the time-varying effect. All models were adjusted for age at index date, sex, educational level, yearly individualized family income, cohabitation status, history somatic diseases, as well as family history of cardiovascular disease (in population comparison).
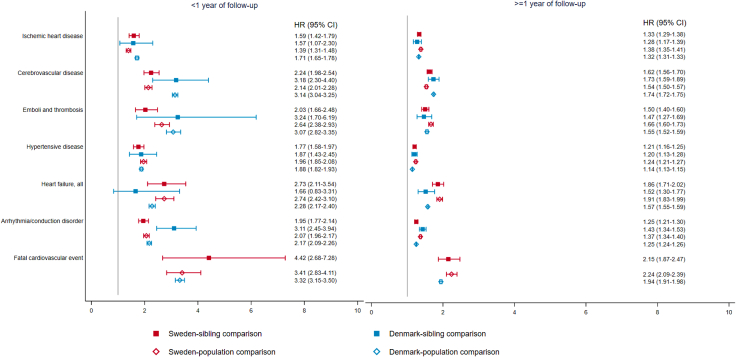
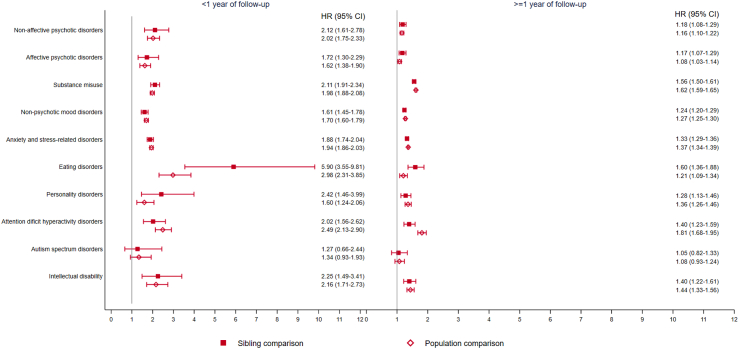
A higher rate was noted for most categories of CVD, including fatal cardiovascular events, within and beyond first year since diagnosis of psychiatric disorders (Fig. 3; Appendix Table S7). We found a higher rate of CVD for most groups of psychiatric disorders except for ASD (Fig. 4), with the greatest rate increase observed for eating disorder, particularly anorexia nervosa (Appendix Table S8). Similar rate increases were observed in all categories of CVD in the Danish cohort (Fig. 3).
Fig. 3.
Hazard ratio with 95% confidence interval of different categories of cardiovascular disease among patients with any psychiatric disorder, compared to their unaffected full siblings (sibling comparison) or matched reference population (population comparison), by time since psychiatric diagnosis (<1 or ≥1 year)∗. ∗Cox models were stratified by family identifiers in sibling comparison and matching identifiers (birth year and sex) in population comparison, controlling for age at index date, sex, educational level, individualized family income, cohabitation status, history of somatic diseases, and family history of cardiovascular disease (in population comparison). Time since index date was used as underlying time scale.
Fig. 4.
Hazard ratio with 95% confidence interval of an incident cardiovascular disease among patients with different types of psychiatric disorders, compared to their unaffected full siblings (sibling comparison) or matched population (population comparison), by time since psychiatric diagnosis (<1 or ≥1 year)∗. ∗Cox models were stratified by family identifiers in sibling comparison and matching identifiers (birth year and sex) in population comparison, controlling for age at index date, sex, education level, individualized family income, cohabitation status, history of somatic diseases, and family history of cardiovascular disease (in population comparison). Time since index date was used as underlying time scale.
Disease trajectory analysis
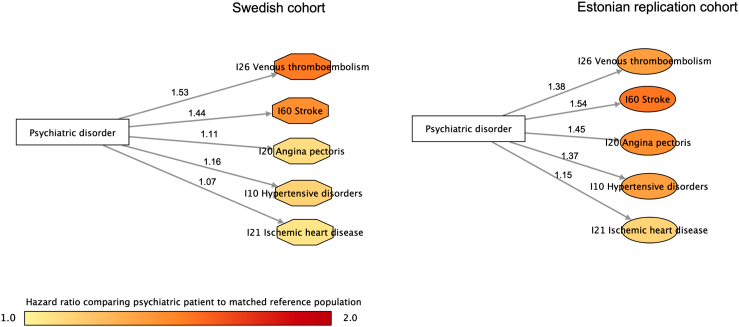
We identified 42 disease trajectories linking psychiatric disorders with subsequent CVD (Appendix Section 4). We found a direct disease pathway between psychiatric disorders and subsequent to hypertensive disorder, ischemic heart disease, venous thromboembolism, angina pectoris, and stroke (i.e., no intermediate diseases identified between these two) (Appendix Figure S3). These pathways were all validated in EstBB with largely similar magnitude of the associations (Fig. 5). Mediated by other medical conditions, we also found an indirect disease pathway between psychiatric disorders and heart failure, cardiac arrhythmia, endocarditis, cardiomyopathy, mediated through different medical conditions (Appendix Figure S3). When analyzing subgroups of psychiatric disorders, we found overall similar pattern of results, although lower number of disease pathways were observed (Appendix Figure S4).
Fig. 5.
Disease trajectories with direct link between psychiatric disorders and incident cardiovascular disease, with hazard ratios derived from the Swedish cohort and Estonian replication cohort∗. ∗This figure illustrates disease trajectories with direct link between psychiatric disorders and incident cardiovascular disease. The ICD codes used to cluster medical conditions are shown in circle. The color of each circle represents the magnitude of the association (hazard ratio) between psychiatric disorders and each cardiovascular disease. The number on each arrow corresponds to the hazard ratio of the association between the two medical conditions of each disease pair.
Discussion
Leveraging a large nationwide cohort of two countries including virtually all patients diagnosed with a psychiatric disorder over a 30-year period, their unaffected full siblings and a set of randomly selected population comparison groups, our study demonstrates a robust association between psychiatric disorders and subsequent risk of incident CVD, independent of familial factors and somatic comorbidities. The highest rate increment was noted during the first year after diagnosis, indicating a high-risk time window for clinical surveillance. Further, we identified a total of 42 disease trajectories linking psychiatric disorders to CVD, including a direct link between psychiatric disorders and hypertensive disorders, ischemic heart disease, venous thromboembolism, angina pectoris, and stroke. The associations were replicated in a Danish nationwide cohort whereas the disease trajectories were validated in the Estonian Biobank.
Our study is the first to comprehensively assess the association between all common psychiatric disorders and CVD with vigorous control of familial factors and comorbidities. Our findings confirm the results from the existing literature, suggesting a positive association between specific psychiatric disorders and CVD. For instance, previous studies and several meta-analyses have reported an increased risk of selected CVDs among patients with depression,30 anxiety,31 and PTSD.32 With few exceptions,13,14,33 these studies have, however, not addressed the role of familial factors in the associations. Previous studies have reported a positive association between obsessive-compulsive disorder as well as stress-related disorders and CVD, independent of familial confounding.13,14,33 Our study therefore extends the existing knowledge base by demonstrating robust associations, between a wide range of psychiatric disorders and subsequent risk of incident CVD and fatal CVD events, independent of shared familial factors. We further showed that the excess rates of CVD after diagnosis of psychiatric disorders was persistent up to 30 years, although the highest risk increase was observed during the first year. Increased medical surveillance may partly explain the risk increase for some of the CVD outcomes, e.g., hypertension, during the immediate time period after diagnosis of psychiatric disorders. It is however unlikely to explain the risk increase of most other acute and severe CVD outcomes which often require immediate medical attention and of fatal CVD events.
In the present study, the association between psychiatric disorders and CVD was noted in men and women, across all age groups and calendar periods, and among individuals with or without a history of somatic diseases and family history of CVD. We found an increased risk of incident CVD among most groups of psychiatric patients, except for patients with ASD, possibly due to the young age at diagnosis of ASD (12 years in the present study). Patients with eating disorders, in particular anorexia nervosa, were found to have the greatest risk increase of CVD, likely due to malnutrition in anorexia nervosa and the cardiovascular implications of energy deprivation and starvation.34
We found a direct link between psychiatric disorders and hypertensive disorder, ischemic heart disease, venous thromboembolism, angina pectoris, and stroke. The underlying mechanisms of a higher risk of these CVDs following a psychiatric disorder may involve multiple pathological processes. The increased risk noted immediately after a diagnosis of psychiatric disorders may indicate a direct impact of stress-induced hypothalamic activation and changes in sympathetic nervous system, which might contribute to vasoconstriction and increased peripheral vascular resistance, leading to high blood pressure, high heart rate, and low heart rate variability.35 The inflammatory responses subsequent to severe stress reaction may also promote atherosclerotic formation,35 contributing to thromboembolism. Psychological stress can also play an important role in individuals with high atherosclerotic plaque burden,36 triggering, for example, acute cardiac events.36 In addition, psychotropic medications, e.g., antidepressants, have been shown to have vasoconstrictive effect37 and complications of QT prolongation.38 The health behaviors of individuals with psychiatric disorders can as well contribute to the hyperadrenergic state, leading to vasoconstriction and tachycardia.39 Other lifestyle factors previously associated with psychiatric disorders, such as physical inactivity, smoking, and unhealthy diet, are known risk factors for CVD.40 In the present study, we also identified several disease pathways that are mediated through other medical conditions, such as sepsis and autoimmune arthritis, suggesting a dysregulation of immune system among patients with psychiatric disorders.41 These mechanisms may interact in CVD development after diagnosis of psychiatric disorders both in the short and long run. Regardless, because the disease trajectory analysis captured only two medical conditions following psychiatric disorders, per trajectory, the role of mediating conditions should be further studied.
The strengths of our study include the use of data collected prospectively in a nationwide sample and the complete follow-up, largely minimizing selection and information biases. The sibling comparison alleviates concerns of familial confounding shared between siblings. The nationwide coverage of high-quality health register data complemented with the large sample size enabled comprehensive exploration on various medical conditions to identify disease trajectories following diagnosis of psychiatric disorder. The validation of the observed associations and disease trajectories in two distinct populations lends further support to the robustness and generalizability of our findings.
There are some limitations to be acknowledged. First, psychiatric disorders and CVD were identified through clinical diagnosis from specialized care in Sweden. This likely has led to underestimation of patients with psychiatric disorders and CVD, e.g., patients attending in primary care only. Yet, the replication of the findings in Denmark, where diagnoses from primary care are included, does not suggest a substantial influence of this misclassification on the reported associations. The register diagnoses have not been validated for all the studied psychiatric disorders and CVDs. However, previous validation studies have shown good to excellent validity of the diagnoses in both Sweden42,43 and Denmark.44,45 Further, given the long study period, the diagnostic criteria for psychiatric disorders and some CVDs might have changed over time. However, as we observed an increased risk of CVD during the up to 30 year follow-up period, we suspect that such change is unlikely to substantially bias our results. Regardless, sub-clinical psychopathology is however not captured in the analysis of either Swedish or Danish data, and warrants separate investigations. Second, patients with psychiatric disorders may have more frequent health care consultations and therefore be more likely to be diagnosed with CVD. However, many of the studied CVD outcomes are severe and require immediate medical attention, which leaves little room for potential surveillance bias. Further, it is possible that some medical conditions (e.g., dermatitis and hypotension) of the disease trajectory analysis may be associated with the treatments for psychiatric disorders and CVD, instead of the diseases. This possibility will need to be delineated further. Finally, given the register-based nature of the present study, we cannot exclude the possibility of residual confounding due to unknown and unmeasured confounders. For instance, although we used sibling comparison to alleviate concern on familial confounding, some lifestyle factors (e.g., smoking, diet, and physical exercise) are not necessarily shared between siblings and could therefore not be controlled for in the sibling comparison.
In conclusion, using large nationwide cohorts of two Nordic countries, we found that patients with psychiatric disorders were at a higher risk of CVD compared to their full siblings and matched population reference, particularly during the first year after diagnosis. Our study demonstrated several distinct disease trajectories linking psychiatric disorders to CVD. These findings provide evidence for the importance of clinical management of cardiovascular risk among patients with psychiatric disorders, particularly during the first year after diagnosis, and contribute to a better understanding of the mechanisms underlying the development of CVD following psychiatric disorders. Increased surveillance and treatment of CVDs and CVD risk factors should be considered as an integral part of clinical management of newly diagnosed patients with psychiatric disorders, in order to reduce risk of CVD among patients with psychiatric disorders.
Contributors
QS, FF, and UV were responsible for the study concept and designed the study. QS, DHM and LBL performed the statistical analysis. QS, FF, and UV drafted the manuscript. QS, DHM, LBL, HS, SK, TA, JB, YL, PFS, WY, KF, PT, YP, OAA, AB, LM, FF and UV analyzed and interpreted the data. QS, FF, and UV had full access to and verified the Swedish data, DHM and AB had full access to and verified the Danish data, and LBL, SK, and LM had full access to and verified the Estonian data in the study. All authors contributed to the writing and revision of the manuscript, and approved the final version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. UV is the guarantor.
Data sharing statement
The register data are not publicly available due to privacy protection, including General Data Protection Regulation (GDRP). Access to Swedish and Danish register resources is only granted after ethical review by appropriate authorities. Further information on data access to Swedish register data can be found at the Swedish National Board of Health and Welfare (https://bestalladata.socialstyrelsen.se/, email: registerservice@socialstyrelsen.se) and/or Statistics Sweden (https://www.scb.se/vara-tjanster/bestall-data-och-statistik/, email: scb@scb.se), and to Danish register data can be found at https://www.dst.dk/en/TilSalg/Forskningsservice, or by contacting the senior corresponding authors. Access to data from the Estonian Biobank can only be requested directly from the Estonian Biobank (releases@ut.ee, https://genomics.ut.ee/en/content/estonian-biobank) upon approval by its scientific committee and the Estonian Committee on Bioethics and Human Research.
Declaration of interests
OAA declares receiving grants or contracts from the South-East Norway Health Authority, NIH and KG Jebsen Stiftelsen, receiving consulting fees from Biogen, Cortechs.ai and Milken. OAA gets Speaker's honorarium from Janssen, Lundbeck and Sunovion, and has a patent on Intranasal Administration (US20160310683 A1). OAA participated in advisory board as National PI for JANSSEN trial depression, MAPS trial PTSD and BI trial schizophrenia. OAA declares having stock at Cortechs.ai. UAV declares receiving support from EPA2023, ISTSS2022 as keynote speaker, and serves on a NordForsk expert committee on Long COVID. FF declares receiving grants from the Swedish Research Council, Swedish Research Council for Health, Workinglife and Welfare, Swedish Cancer Society, European Research Council and the US CDC. FF declares getting payment from Swedish Research Council for grant review. All other authors declare no competing interests.
Acknowledgements
This research was supported by EU Horizon 2020 Research and Innovation Action Grant (CoMorMent, Grant nr. 847776 to UAV, PFS, OAA and FF); European Research Council Consolidator grant (StressGene, Grant nr. 726413 to UAV); Icelandic Research fund (to UAV); Swedish Research Council (Vetenskapsrådet, award D0886501 to PFS); US NIMH R01 MH123724 to PFS; the Outstanding Clinical Discipline Project of Shanghai Pudong (Grant No.: PWYgy2021-02) and the Fundamental Research Funds for the Central Universities (to QS); the European Union through the European Regional Development Fund (Project No. 2014-2020.4.01.15-0012 to LM); the Research Council of Norway (RCN grants 223273, 296030, 300309, 324252 to OAA); the South-East Regional Health Authority (grant 2017-112, 2022-073 to OAA); the Stiftelsen Kristian Gerhard Jebsen (grants SKGJ-MED-008 and SKGJ-MED-021 to OAA); and the EEA-RO-NO-2018-0535 (to OAA).
We would like to thank Andres Metspalu and Tõnu Esko for their contribution in data collection for the Estonian Biobank research team.
UV affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102063.
Contributor Information
Qing Shen, Email: qing.shen@ki.se.
Unnur Valdimarsdóttir, Email: unnurav@hi.is.
Appendix A. Supplementary data
References
- 1.Momen N.C., Plana-Ripoll O., Agerbo E., et al. Association between mental disorders and subsequent medical conditions. N Engl J Med. 2020;382:1721. doi: 10.1056/NEJMoa1915784. published online April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker E.R., McGee R.E., Druss B.G. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72:334–341. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roest A.M., Martens E.J., de Jonge P., Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Boscarino J.A. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med. 2008;70:668–676. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White J.R., Chang C.C.H., So-Armah K.A., et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: veterans aging cohort study. Circulation. 2015;132:1630–1638. doi: 10.1161/CIRCULATIONAHA.114.014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khambaty T., Stewart J.C., Gupta S.K., et al. Association between depressive disorders and incident acute myocardial infarction in human immunodeficiency virus-infected adults: veterans aging cohort study. JAMA Cardiol. 2016;1:929–937. doi: 10.1001/jamacardio.2016.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zivin K., Yosef M., Miller E.M., et al. Associations between depression and all-cause and cause-specific risk of death: a retrospective cohort study in the Veterans Health Administration. J Psychosom Res. 2015;78:324–331. doi: 10.1016/j.jpsychores.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Ebrahimi R., Lynch K.E., Beckham J.C., et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6:642–651. doi: 10.1001/jamacardio.2021.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patten S.B., Williams J.V.A., Lavorato D.H., Modgill G., Jetté N., Eliasziw M. Major depression as a risk factor for chronic disease incidence: longitudinal analyses in a general population cohort. Gen Hosp Psychiatry. 2008;30:407–413. doi: 10.1016/j.genhosppsych.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Almas A., Moller J., Iqbal R., Lundin A., Forsell Y. Does depressed persons with non-cardiovascular morbidity have a higher risk of CVD? A population-based cohort study in Sweden. BMC Cardiovasc Disord. 2019;19:260. doi: 10.1186/s12872-019-1252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whang W., Kubzansky L.D., Kawachi I., et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses' Health Study. J Am Coll Cardiol. 2009;53:950–958. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C.J., Hsieh M.H., Hou W.H., Liu J.C., Jeng C., Tsai P.S. Depression, antidepressants, and the risk of coronary heart disease: a population-based cohort study. Int J Cardiol. 2013;168:4711–4716. doi: 10.1016/j.ijcard.2013.07.173. [DOI] [PubMed] [Google Scholar]
- 13.Isomura K., Sidorchuk A., Brander G., et al. Risk of specific cardiovascular diseases in obsessive-compulsive disorder. J Psychiatr Res. 2021;135:189–196. doi: 10.1016/j.jpsychires.2020.12.066. [DOI] [PubMed] [Google Scholar]
- 14.Song H., Fang F., Arnberg F.K., et al. Stress related disorders and risk of cardiovascular disease: population based, sibling controlled cohort study. BMJ. 2019;365 doi: 10.1136/bmj.l1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rødevand L., Bahrami S., Frei O., et al. Polygenic overlap and shared genetic loci between loneliness, severe mental disorders, and cardiovascular disease risk factors suggest shared molecular mechanisms. Transl Psychiatry. 2021;11:1–11. doi: 10.1038/s41398-020-01142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelishadi R., Poursafa P. A review on the genetic, environmental, and lifestyle aspects of the early-life origins of cardiovascular disease. Curr Probl Pediatr Adolesc Health Care. 2014;44:54–72. doi: 10.1016/j.cppeds.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Rokita K.I., Dauvermann M.R., Donohoe G. Early life experiences and social cognition in major psychiatric disorders: a systematic review. Eur Psychiatry. 2018;53:123–133. doi: 10.1016/j.eurpsy.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 18.D'Onofrio B.M., Lahey B.B., Turkheimer E., Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health. 2013;103(Suppl 1):S46–S55. doi: 10.2105/AJPH.2013.301252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steptoe A., Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 20.Jensen A.B., Moseley P.L., Oprea T.I., et al. Temporal disease trajectories condensed from population-wide registry data covering 6.2 million patients. Nat Commun. 2014;5:4022. doi: 10.1038/ncomms5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H., Pawitan Y., He W., et al. Disease trajectories and mortality among women diagnosed with breast cancer. Breast Cancer Res. 2019;21:95. doi: 10.1186/s13058-019-1181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han X., Hou C., Yang H., et al. Disease trajectories and mortality among individuals diagnosed with depression: a community-based cohort study in UK Biobank. Mol Psychiatry. 2021;26:1–11. doi: 10.1038/s41380-021-01170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludvigsson J.F., Andersson E., Ekbom A., et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekbom A. In: Methods in biobanking. Dillner J., editor. Humana Press; Totowa, NJ: 2011. The Swedish multi-generation register; pp. 215–220. [Google Scholar]
- 25.Richardson D.B. An incidence density sampling program for nested case-control analyses. Occup Environ Med. 2004;61:e59. doi: 10.1136/oem.2004.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nevriana A., Pierce M., Dalman C., et al. Association between maternal and paternal mental illness and risk of injuries in children and adolescents: nationwide register based cohort study in Sweden. BMJ. 2020;369 doi: 10.1136/bmj.m853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Statistikmyndigheten. Longitudinell integrationsdatabas för sjukförsäkrings- och arbetsmarknadsstudier (LISA). Stat. Cent. 2021. https://www.scb.se/vara-tjanster/bestall-data-och-statistik/bestalla-mikrodata/vilka-mikrodata-finns/longitudinella-register/longitudinell-integrationsdatabas-for-sjukforsakrings--och-arbetsmarknadsstudier-lisa/
- 28.Lambert P.C., Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9:265–290. [Google Scholar]
- 29.Leitsalu L., Haller T., Esko T., et al. Cohort profile: Estonian Biobank of the Estonian Genome Center, University of Tartu. Int J Epidemiol. 2015;44:1137–1147. doi: 10.1093/ije/dyt268. [DOI] [PubMed] [Google Scholar]
- 30.Van der Kooy K., van Hout H., Marwijk H., Marten H., Stehouwer C., Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 31.Batelaan N.M., Seldenrijk A., Bot M., van Balkom A.J.L.M., Penninx B.W.J.H. Anxiety and new onset of cardiovascular disease: critical review and meta-analysis. Br J Psychiatry. 2016;208:223–231. doi: 10.1192/bjp.bp.114.156554. [DOI] [PubMed] [Google Scholar]
- 32.Edmondson D., von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4:320–329. doi: 10.1016/S2215-0366(16)30377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaccarino V., Goldberg J., Rooks C., et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62:970–978. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sachs K.V., Harnke B., Mehler P.S., Krantz M.J. Cardiovascular complications of anorexia nervosa: a systematic review. Int J Eat Disord. 2016;49:238–248. doi: 10.1002/eat.22481. [DOI] [PubMed] [Google Scholar]
- 35.Osborne M.T., Shin L.M., Mehta N.N., Pitman R.K., Fayad Z.A., Tawakol A. Disentangling the links between psychosocial stress and cardiovascular disease. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.120.010931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kivimäki M., Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15:215–229. doi: 10.1038/nrcardio.2017.189. [DOI] [PubMed] [Google Scholar]
- 37.Marano G., Traversi G., Romagnoli E., et al. Cardiologic side effects of psychotropic drugs. J Geriatr Cardiol. 2011;8:243–253. doi: 10.3724/SP.J.1263.2011.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beach S.R., Celano C.M., Sugrue A.M., et al. QT prolongation, Torsades de Pointes, and psychotropic medications: a 5-year update. Psychosomatics. 2018;59:105–122. doi: 10.1016/j.psym.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Akasaki Y., Ohishi M. Cerebrovascular and cardiovascular diseases caused by drugs of abuse. Hypertens Res. 2020;43:363–371. doi: 10.1038/s41440-019-0367-7. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen R.E., Banner J., Jensen S.E. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol. 2021;18:136–145. doi: 10.1038/s41569-020-00463-7. [DOI] [PubMed] [Google Scholar]
- 41.Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98:477–504. doi: 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellgren C., Landén M., Lichtenstein P., Hultman C.M., Långström N. Validity of bipolar disorder hospital discharge diagnoses: file review and multiple register linkage in Sweden: validity of register-based bipolar disorder. Acta Psychiatr Scand. 2011;124:447–453. doi: 10.1111/j.1600-0447.2011.01747.x. [DOI] [PubMed] [Google Scholar]
- 43.Ekholm B., Ekholm A., Adolfsson R., et al. Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59:457–464. doi: 10.1080/08039480500360906. [DOI] [PubMed] [Google Scholar]
- 44.Uggerby P., Østergaard S.D., Røge R., Correll C.U., Nielsen J. The validity of the schizophrenia diagnosis in the Danish Psychiatric Central Research Register is good. Dan Med J. 2013;60:A4578. [PubMed] [Google Scholar]
- 45.Nissen J., Powell S., Koch S.V., et al. Diagnostic validity of early-onset obsessive-compulsive disorder in the Danish Psychiatric Central Register: findings from a cohort sample. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.