Abstract
Longitudinal studies have demonstrated that altered indices of airway function, assessed shortly after birth, are a risk factor for the subsequent development of wheezing illnesses and asthma, and that these indices predict airway size and airway wall thickness in adult life. Pre- and post-natal factors that directly alter early airway function such as extreme prematurity and cigarette smoke may continue to affect airway function and hence the risks for wheeze and asthma. Early airway function and an associated asthma risk may also be indirectly influenced by immune system responses, respiratory viruses, the airway microbiome, genetics and epigenetics, especially if they affect airway epithelial dysfunction. Few if any interventions, apart from smoking avoidance, have been proven to alter the risks of developing asthma, but vitamin C supplementation to pregnant smokers my help decrease the effects of in utero smoke on offspring lung function. We conclude that airway size and the factors influencing this play an important role in determining the risk for asthma across the lifetime. Progress in asthma prevention is long overdue and this may benefit from carefully designed interventions in well phenotyped longitudinal birth cohorts with early airway function assessments monitored through to adulthood.
Keywords: lung function, airway function, prenatal determinants, asthma, wheeze
Introduction
The origins of many of the phenotypes of asthma occur during the fetal and early childhood period and children who develop asthma by age seven have been shown to have deficits in lung function as neonates (1). This commentary will summarize the role of lung function, as an index of airway functional size, as a risk factor for the subsequent development of wheezing illnesses and asthma. The commentary will also present the prenatal and the early postnatal factors important in determining early airway size and also factors that continue to influence airway function and the subsequent development of asthma. It will also present an example of potential primary prevention with vitamin C supplementation to pregnant smokers to mitigate the adverse effect of in utero smoke on lung development and function.
Prenatal Determinants of Lung Function in Relation to Asthma
Airway disease begins early, through prenatal and early postnatal influences, and through transgenerational effects due to epigenetic changes. This early focus is a paradigm switch for many practitioners who, when seeing a wheezing child often ask about trigger factors and medical compliance, all very important, but not about early life factors such as prematurity and pregnancy exposures, which are key to prevention of asthma. There are multiple prenatal factors that can adversely affect lung development and place the infant on a lower lung function trajectory. This can impact the attainment of maximal lung function plateau in early adulthood and in turn impact their ability to achieve lifelong lung health (2–5). These factors can be broadly classified into often overlapping categories including: maternal environmental exposures/toxins and pregnancy complications, preterm birth with or without bronchopulmonary dysplasia (BPD), maternal nutrition and lifestyle. Early lung function is important in investigating these prenatal factors.
Prenatal Environmental Exposures/Toxins
Prenatal environmental exposures that have been shown to be associated with lung development and lung function in term and preterm infants include exposure to nicotine and tobacco smoke, indoor and outdoor pollution, medication or drug exposure during pregnancy, antenatal inflammation, delivery mode, early postnatal oxygen supplementation and exposure to positive pressure ventilation (3;5). Exposure during pregnancy to various pollutants has been associated with early decreases in the offspring’s spirometry (6;7). Household air pollution during pregnancy was associated with decreased lung function at one month of age and increased risk of pneumonia before one year of age (8).
Pulmonary and airway function testing has demonstrated the significant adverse effect of prenatal tobacco products exposure on lung/airway development and have linked these changes to subsequent childhood respiratory disease (9). This is one of the most potentially preventable insults to the developing lung and can impact lung development from multiple standpoints: pre-clinical data indicates prenatal nicotine exposure increases collagen deposition throughout the lung and airways (10) and increases airway branching among other effects (11); while smoking in pregnancy increases preterm delivery, low birth weight, and intrauterine growth restriction (IUGR) (12). All of the latter are also important factors of altered lung function as shown by lung function testing. Unfortunately, the prevalence of smoking during pregnancy continues to be about 10–12% in the United States and is still high throughout the world (13). World-wide over 50% of pregnant smokers continue during pregnancy (14). These findings are of particular concern with the increasing use of electronic cigarettes (9).
Cohort studies have reported that adverse respiratory outcomes related to in utero smoke exposure track into child and adulthood, with early and persistent decreases in offspring lung function (15–22). Infants (term and preterm) exposed to in utero smoke have decreased pulmonary function at birth that supports anatomic changes, including changes in flow volume characteristics, respiratory system compliance (Crs), and forced expiratory flows (FEFs), even before postnatal smoke exposure (20;23;24). Young children exposed to in utero smoke had 7–10% decreases in forced expiratory volume in one second (FEV1) (19) and infants exposed to in utero smoke had a lower maximal expiratory flow at functional residual capacity (V̇maxFRC) than those not exposed (15). A prospective study of 2400 patients demonstrated decreased FEV1 and other forced expiratory flows to 21 years of age after in utero smoke exposure, the in utero effect being much greater than that of post-natal exposure (22).
Preterm Delivery
Prematurity (birth at < 37 weeks gestation) is the most common cause of altered lung and airway development, as maturation occurs postnatally under altered mechanical and environmental conditions, including active tidal breathing with strain and stretch of immature intrathoracic structures, and a state of relative hyperoxia, even with room air(3;5). Extremely preterm infants born at <28 weeks gestation are at high risk for BPD which is characterized by alveolar hypoplasia and obstructive airway disease (25;26). In addition to prematurity, other prenatal factors such as chorioamnionitis, preeclampsia, pre-existing hypertensive disorders, gestational diabetes, and maternal obesity are associated with an increased risk for BPD (12;27). For instance, a prospective, longitudinal study of 587 preterm infants demonstrated that maternal smoking during pregnancy and maternal hypertension each was associated with a significant two-fold increase in the odds of BPD after preterm birth (12). In infants with severe BPD studied at 55 weeks of postmenstrual age (PMA), three distinct phenotypes of lung function were identified, but 91% of the infants had airway obstruction (28). Serial measurements in BPD have also demonstrated significant reductions in expiratory flows into adulthood that tend to track along the same lung function percentile (29–32).
Lung function testing has demonstrated that healthy infants born only a few weeks prematurely also demonstrate altered lung development compared to term reference infants, with decreased Crs when measured at term PMA (33), and FEFs that remain decreased into childhood (34). This gives a physiologic basis for the increased asthma symptoms these infants demonstrate as a whole as they age, and offers the opportunity for potentially early identification of at risk children. Infants with tidal breathing measurements that were below the median shortly after birth were significantly more likely at 10 years of age to have a history of asthma (35). Lower V̇maxFRC in the first year of life was associated with increased risk of wheezing, as well as an increased risk for active asthma up to age 36 years (36).
Maternal Nutrition/Supplements/Lifestyle
Maternal malnutrition, obesity, and micronutrient intake have all been shown to affect lung development and function. IUGR is a risk factor for decreased lung function in infancy, childhood, and adulthood and although multi-factorial can be associated with poor nutrition. Several studies have demonstrated that term infants with IUGR had lower FEV1 than normally grown age-matched school-aged peers (37;38). An obesogenic intra-uterine environment with increased oxidative stress and increased cytokine production has been associated with increased bronchodilator and steroid dispensing in early childhood (39). Maternal micronutrient intake (including supplemental vitamins A, C, D, and E) play a role in fetal lung development, protect against oxidant damage, are pro-angiogenic factors, and can modulate the inflammatory response that may impact lung development (40). In a double blind, randomized controlled trial (RCT), maternal vitamin A supplementation before, during, and after pregnancy in a malnourished Nepali population was shown to significantly improve the offspring’s spirometry measurements at 9 to 13-years of age (41).
Primary Prevention: Within our Grasp?
As outlined, antenatal events have a significant effect on lung development and therefore childhood asthma. This makes early intervention, at least as early as during prenatal care, a paramount priority if the incidence of asthma is to be decreased. An example of potential primary prevention is vitamin C supplementation in the face of in utero smoke to promote normal lung development.
Based on pre-clinical results in a pregnant primate model, two separate RCTs of vitamin C (500 mg/day) versus placebo to pregnant women unable to quit smoking cigarettes, have demonstrated improved lung/airway function in the offspring of the vitamin C supplemented smokers (23;42;43). The first study randomized 159 pregnant smokers at < 22 weeks gestation, and the vitamin C supplemented offspring had a significant increase in their neonatal tidal breathing parameters and Crs, and a 48% decrease in wheeze through 1-year of age (23). The second RCT randomized 252 pregnant smokers. The offspring of the vitamin C compared to the placebo group had significantly higher FEFs (FEF 25–75, FEF50, FEF75) and FEV0.5 by repeated measures analysis at 3 and 12-months of age (42;43). Follow-up through 5-years of age demonstrated sustained effects with higher FEFs and decreased wheeze at 4–6 years of age in the offspring of the vitamin C supplemented group (Figure 1A and 1B) (44). Vitamin C may be a safe and inexpensive intervention to mitigate the effects of smoking during pregnancy, in addition to continued cessation counseling.
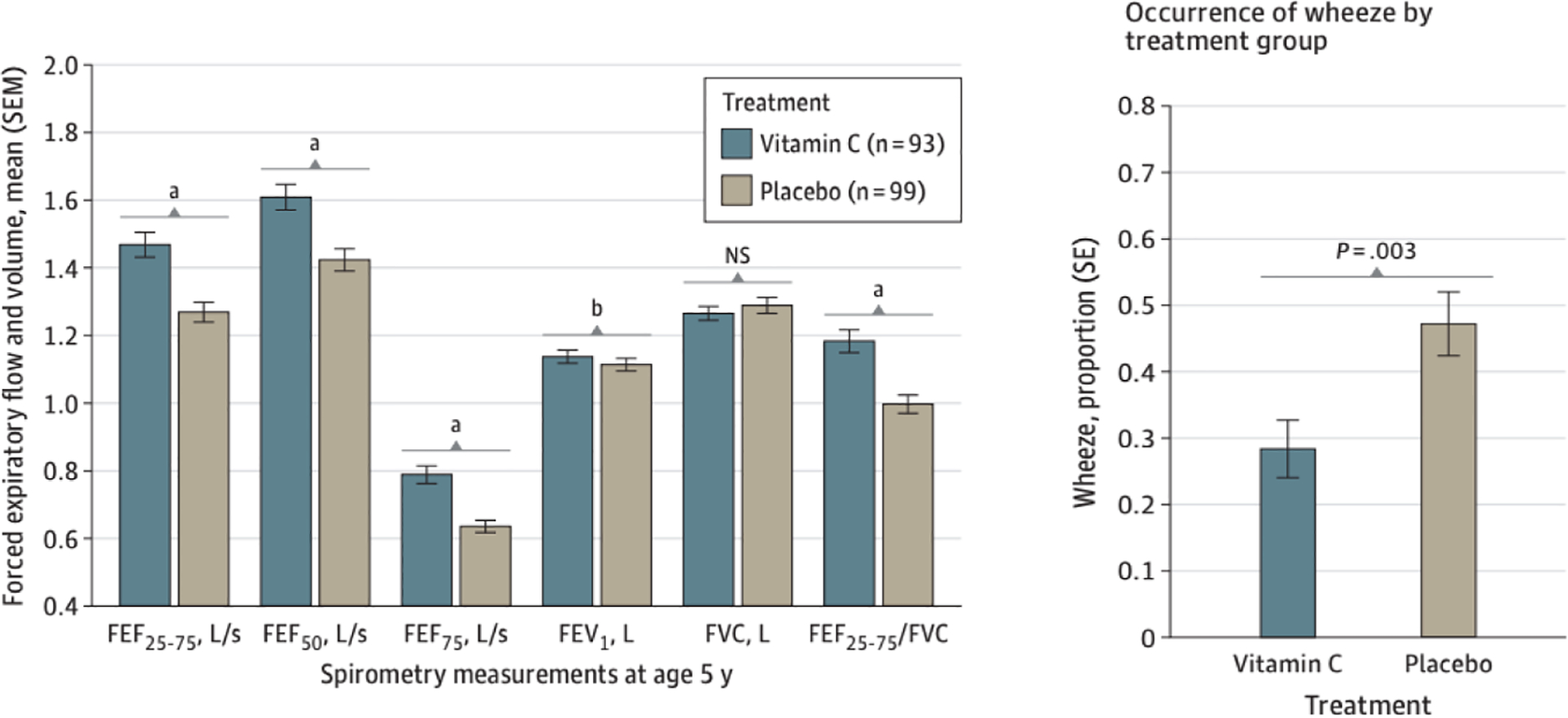
Figure 1A. Airway Function Test Results Obtained by Spirometry Measurements at Age 5 Years in Children Born to Pregnant Smokers Randomized to Vitamin C Supplementation (500mg/d) vs Placebo
Spirometry measurements are shown for offspring of 93 and 99 pregnant smokers in the vitamin C and placebo treatment groups, respectively. P values were adjusted for trial stratification variables of study site and gestational age at randomization, sex, race and ethnicity, and height at testing. All forced expiratory flow (FEF) and forced expiratory volume in 1 second (FEV1) measurements increased significantly in offspring of vitamin C–treated pregnant smokers. FEF25–75 indicates FEF between 25% and 75% expired volume; FEF50 indicates FEF at 50% of expiration; FEF75, FEF at 75% of expiration; and NS, not significant. a P < .01; b P < .05.
Figure 1B. Any Occurrence of Wheeze between the Fourth and Sixth Birthday in Offspring of Pregnant Smokers Randomized to Vitamin C versus Placebo during Pregnancy
Multiple logistic regression was used to compare the occurrence of wheeze between the vitamin C and placebo groups adjusting for study trial design factors of study site and gestational age at randomization, and covariates of race, and sex, and significant two-way interactions of all of these variables. 212 children were included (106 vitamin C and 106 placebo treated). The children born to the vitamin C treated pregnant smokers had a significant decrease in current wheeze at 28% versus 47% (estimated OR of 0.41; 95% CI: 0.23–0.74; P= 0.0032). (Reproduced from Jama Pediatr. 2023:177(1):16–24 with permission).
Fetal origins of asthma in children born at term
As a group, patients with asthma have lower level of airway function than those without asthma. This is particularly true for asthma patients in the severe end of the spectrum, but also, to a lesser extent, for those with mild disease (45). Longitudinal studies have shown decreased growth in airway function in children (46;47) and increased lung function decline in adults with asthma, especially among those with recurrent exacerbations (48). The steepest deficits have been observed during the first years of life (Figure 2). A potential explanation for this association is that chronic airway inflammation, especially of the eosinophilic type, induces airway remodeling and, by this mechanism, causes airflow limitation. Cross-sectional studies showed that patients with asthma who had evidence of airflow limitation have increased proportion of sputum eosinophils as compared with those without airflow limitation (45). In a recent longitudinal study of asthma patients, Tang et al (49) reported that worsening of mucus plugs in computed tomography (CT) lung scans after three years of follow up was associated with increase in airflow obstruction, and the presence of mucus plugs correlated with sputum eosinophilia. Taken together, these findings suggest that airflow limitation in asthma can be caused by the disease process itself, especially during the first years of life.
Figure 2. Changes in Lung Function During the Course of Mild and Moderate Asthma.
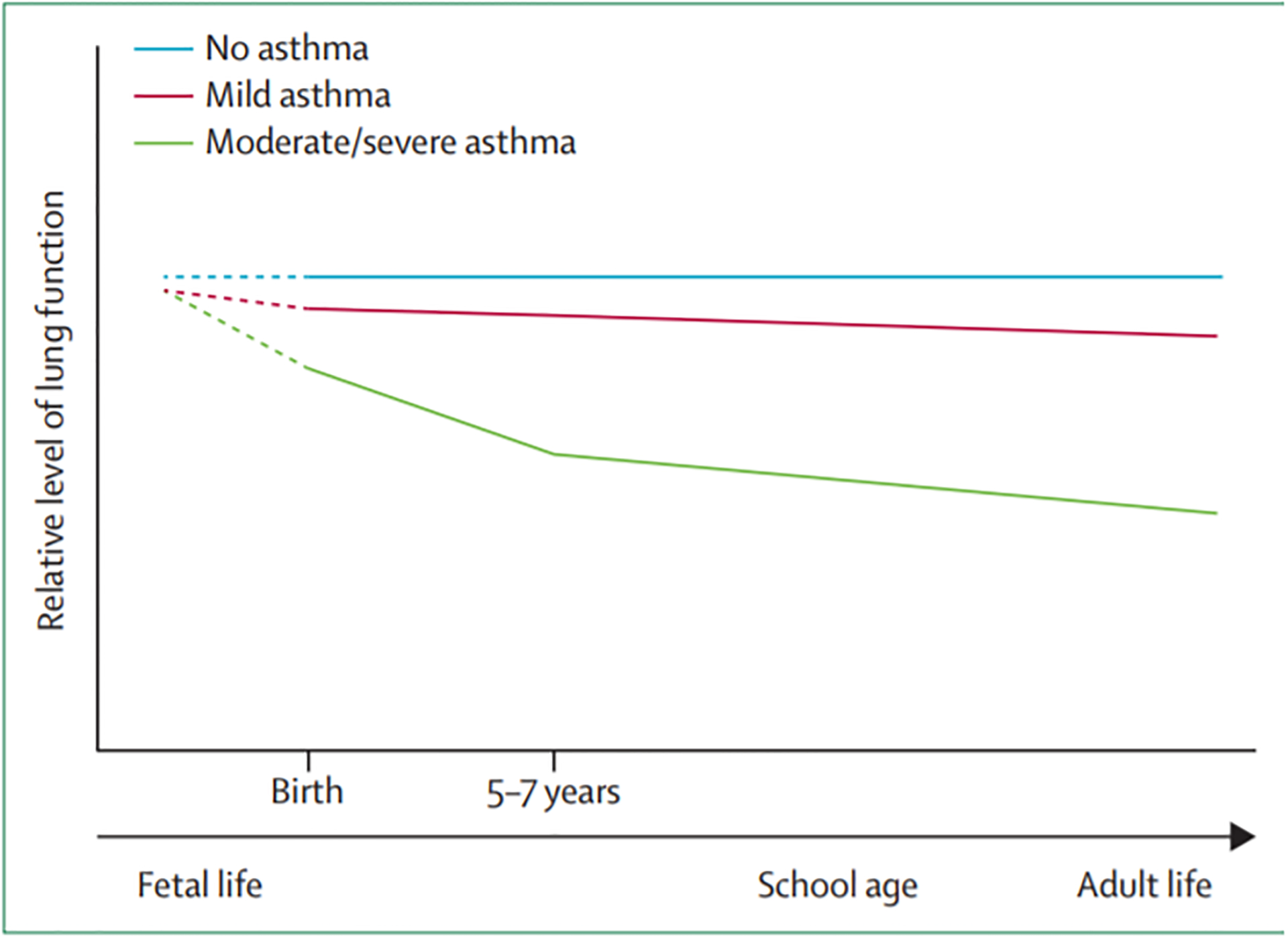
Schematic representation of airway function trajectories for children without asthma, with asthma and mild or no airflow limitation, and for children with persistent asthma and airflow limitation. As a group, the latter show impaired airways function growth especially during the preschool years. These deficits extend, albeit in a less evident way, to the school age and, as steeper decline in airway function, to adulthood (Reproduced from Lancet 2013 Oct 19;382(9901):1360–72 with permission).
If alterations in airway function and, presumably, airway structure, may precede the development of asthma and predispose for its subsequent incidence has been the matter of intense scrutiny. Since in a high proportion of cases of asthma, the first symptoms occur during early life (50), testing such hypothesis requires long-term prospective studies in which airway function is assessed before the development of any symptoms, preferably during infancy. Results for several such studies are now available.
Haland et al (35) first reported that Norwegian children whose fraction of expiratory time to peak tidal expiratory flow to total expiratory time [tPTEF/tE] obtained from tidal breathing flow-time loops shortly after birth was at or below the median were more likely to have a history of asthma by 10 years of age than those whose tPTEF/tE was above the median. The tPTEF/tE ratio measured during the first months of life had been previously shown to predict wheezing lower respiratory illnesses by age one in the Tucson Children’s Respiratory Study (TCRS) (51) and is an indirect index of airway obstruction (52). Subsequently, Hallas et al (53) showed in Danish children that airway function, as assessed by maximal mid-expiratory flows (MMEF), was significantly diminished from birth up to age 13 years in children who had asthma by that age. Using data from the Perth Infant Asthma Follow up study, Owens et al (54) showed that infants who had a V̇maxFRC at age 1 month that was below the median were 3.5–4 times more likely to have active asthma at ages 18 and 24 years than those who had V̇maxFRC above the median. More recently, Guerra et al. assessed the association of both tPTEF/tE and V̇maxFRC measured during the first months of life in the TCRS and the subsequent development of asthma (36). They reported that one standard deviation decreases in infant tPTEF/tE and V̇maxFRC were associated with a 70% and 55% increased risk of active asthma between the ages of 6 and 36 years, respectively. These effects were partly independent from each other, and two out of three infants who were in the lowest tertile for both tPTEF/tE and V̇maxFRC developed active asthma by mid-adult life. These results suggest that tPTEF/tE and V̇maxFRC sense different functional characteristics of the infant airway, which can separately contribute to subsequent asthma risk.
To determine if physical characteristics of the airway could be related to lower levels of tPTEF/tE or V̇maxFRC in infancy, Guerra et al correlated these indices with measurements obtained at age 26 years from high resolution computed tomography (HRCT) of airway generations 0 to 10, ranging in size from approximately 14 to 2 mm in diameter (36). No consistent associations were observed between V̇maxFRC in infancy and HRCT-derived measurements. In contrast, low infant tPTEF/tE in infancy was associated with significant reductions in inner diameter, outer diameter, total area, luminal area, and wall area measured at functional residual capacity, but not at total lung capacity, indicating smaller, thinner airways.
These results suggest that tPTEF/tE senses a physical airway feature, namely, potentially more collapsible large and mid-size bronchi, and it can be assumed that this feature could derive in clinical manifestations of airway obstruction if associated, for example, with acute respiratory illnesses. Interestingly, Pekka Malmberg et al (55) found no significant decrease in tPTEF/tE after methacholine inhalation in infancy, suggesting that this measurement is not sensitive to changes induced by airway smooth muscle contraction. On the other hand, given that no consistent anatomical changes were observed in large/mid-size airways of adults who had low V̇maxFRC in infancy, it is plausible to speculate that these individuals have either an inflammatory phenotype or perhaps functional (e.g., bronchial hyperresponsiveness) and/or anatomical changes in their smaller airways, which at present cannot be assessed by use of HRCT. In support of these contentions, Chawes et al (56) reported that neonates enrolled in the Danish birth cohort quoted earlier and who had diminished maximal flows at birth had elevated serum markers of systemic inflammation, including IL-6 and C-reactive protein, during the first year of life. In addition, Pekka Malmberg et al (55) found a strong reduction in V̇maxFRC after methacholine inhalation, and Kotaniemi-Syrjänen et al (57) reported significant correlations between baseline V̇maxFRC and the degree of bronchial hyperresponsiveness observed in infants.
Taken together, these findings suggest that structural and functional characteristics of both large and small airways already present at birth can predispose for the development of asthma symptoms at least up to mid adult life. They also question the assumption that excessive airway inflammation is the only determining factor in all patients with asthma.
The role of the immune system in determining early airway function and subsequent asthma
Immune responses are involved both pre- and postnatally in determining the intensity and direction of inflammation in the developing respiratory system (58;59). Based on the results of early airway functions studies, these responses can be expected to influence ongoing airway function and the development of asthma in children. Distinct differences in immune system responses have long been recognized between those who develop asthma and those that don’t. Early observations that eosinophilia was commonly associated with asthma in children (60) led to the view that atopy was a causal factor for developing airway disease and asthma. Later, atopy was found to be due to over-production of IgE (61) resulting from increased T helper 2 (Th2) responses.
More recent studies have shown that increases in Th2 responses in atopics are commonly associated with decreases in Th1 and innate immune responses that result in reduced interferon responses (62). The relative roles of these T helper responses in determining airway function and asthma in children is still unclear. On one hand, the increased IgE levels in children with increased Th2 responses are plausibly associated with long-term, antigen-induced, IgE-mediated inflammation of the airway wall beginning at an early age (63). However, interferon responses are crucial for protection against respiratory viruses, so the associated decrease in Th1 responses is likely to explain why respiratory virus infections are the main cause of acute respiratory exacerbations associated with wheezing from early in life and in asthmatics (64;65). Curiously, the Th2 system is not known to have an important role in protection from viral infections, but treatment with an anti-IgE antibody such as omalizumab has been shown to decrease acute virus-induced asthma exacerbations (66). This may be because respiratory viruses such as rhinovirus take advantage of the presence of IgE bound to IgE receptors (67) to produce an increase in acute airway inflammation.
These observations lead to the question of whether repeated acute respiratory viral infections in early life cause long-term airway inflammation and damage that predisposes to the reduced airway function associated with asthma and may indeed predispose to asthma. This question has proven remarkably difficult to settle, as associations are common, but proven mechanisms are not. In the Childhood Origins of Asthma (COAST) cohort, the odds ratio of developing asthma by 6 years in children who had a rhinovirus-induced wheezing episode before 3 years of age was 9.8 which was over 3 times higher than the odds ratio for early RSV infection in at-risk children (68). The greatly increased association between early rhinovirus infection versus early respiratory syncytial virus (RSV) infection has been confirmed in a recent meta-analysis (69). Atopy interacts with respiratory virus infections to increase the risk of asthma, (70) but the mechanism of this interaction is still unclear. There is evidence that rhinovirus infection in early life can alter subsequent Th1 and Th2 responses. For example, mice exposed to early-life infection with rhinovirus show augmented IL-25-driven Th2 responses (71). Also, recent evidence has shown that acute infections with RSV reprogram the airway epithelium to alter the response to subsequent infections, (72) but whether this results in on-going airway damage is still not clear. Taken collectively, the data on respiratory viruses suggests that repeated infections may compromise the airway and contribute to asthma, but the absence of well-defined mechanisms and the many potential confounders leave the question of causality unanswered.
The role of the airway microbiome in determining airway function and disease
The role of the airway microbiome in airway function and the development of asthma has not been established despite the observation that bacteria are more common in the airway of neonates who later develop asthma being made 15 years ago (73). The question is whether these bacteria themselves cause airway inflammation leading to asthma (74) or they are simply bystanders due to the known immune system differences between asthmatics and non-asthmatics. In infants who had a nasopharyngeal sample taken during the first year of life, most were colonized with staphylococcus or corynebacterium but subsequent asymptomatic colonization with streptococcus predicted later asthma (75). Azithromycin given to children at-risk of severe lower respiratory infections may reduce the severity of exacerbations, (76) but whether this is due to the known anti-inflammatory action of this drug or its action as an antibiotic has not been ascertained. In children sampled at the time of an acute asthma exacerbation compared with controls, minimal differences were found in the airway microbiome (77). Finally, rhinovirus infections can promote pathogen-dominated airway microbiota that may increase the risk for wheezing (63;78). Overall, the question regarding causality of the airway microbiome in producing sufficient airway inflammation to alter respiratory function or produce asthma has not been resolved.
Genetic and epigenetic causes of airway dysfunction and asthma
Pre- and post-natal airway growth and development is influenced by a wide range of genetic and epigenetic factors involved in innate and adaptive immunity (58;79). For example, airway dysfunction in asthma may involve asthma susceptibility alleles in bronchial epithelial cells (80). Risk alleles in IL1RL1, IL33, TSLP, CDHR3, MUC5AC, KIF3A, EFHC1 and GSDMB have been implicated and suggest a central role of the airway epithelium in reduced pulmonary function and the genesis of asthma (80). Epigenetic changes are also involved in airway epithelial dysfunction in asthmatics (80) and these are likely to be highly responsive to environmental exposures. A recent study showed that more than half of the asthma-associated alleles in the airway epithelium showed evidence of CpG methylation(81)including CDHR3 in children (82).
Glutathione S-transferase (GST) genes are involved in detoxification and this explains their role in the in utero susceptibility to the products of cigarette smoke (83;84). In the Perth Infant Asthma Follow-up birth cohort study, in infants exposed to in utero smoke, infant and/or maternal GSTT1 null was associated with increased airway responsiveness and decreased V̇ maxFRC at 1 month of age and maternal GSTP1 Val/Val or Ile/Val was associated with increased V̇ maxFRC at 6 months of age (85). At 24 years of age, GSTM1-null homozygosity was associated with lower FEV1 and FVC in those with versus those without in utero tobacco exposure (86). These data show that genetically inactive GST genes in the mother or child are risk factors for reduced pulmonary function throughout childhood into adulthood. The GST null genotypes are also associated with an increased risk of asthma in childhood but not in adults (87). Collectively these studies and many others (83;84;88;89) show that GST genes are required for protection from the toxic effects of tobacco products and when they are genetically dysfunctional, children’s airways are compromised and the risk of asthma is increased.
Conclusion
There is now strong evidence suggesting that airway size plays an important role in determining the risk for the development of asthma across the lifetime. Continued advancement in the prevention of asthma is likely to require interdisciplinary collaboration including basic science to define mechanisms and translational research to test hypotheses arising from this. Future interventional clinical translation studies will benefit from being done in well-phenotyped longitudinal birth cohorts following lung function trajectories from soon after birth to adulthood.
Funding:
CTM receives funding from the National Heart, Lung, and Blood Institute R01HL105447, R33HL147906 and the National Institutes of Health UH3OD023288. PNL receives funding from National Health and Medical Research Council (NHMRC) grants #941222, #991309, #404033 and #1031605. FDM was funded by grants R01HL132523 and UH3HL147016 from the National Heart, Lung, and Blood Institute (NHLBI) and P01AI148104 from the National Institute of Allergy and Infectious Diseases.
Abbreviations:
- IUGR
intrauterine growth restriction
- RCTs
randomized controlled trials
- Crs
respiratory system compliance
- FEFs
forced expiratory flows
- V̇maxFRC
maximal expiratory flow at functional residual capacity
- FEV1
forced expiratory volume in one second
Footnotes
Conflict of Interest: The authors have no conflicts of interest or affiliations with companies that have direct financial interests in the subject matter of this article.
Reference List
- (1).Bisgaard H, Jensen SM, Bonnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med 2012. Jun 1;185(11):1183–9. [DOI] [PubMed] [Google Scholar]
- (2).Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med 2019. Apr;7(4):358–64. [DOI] [PubMed] [Google Scholar]
- (3).Jordan BK, McEvoy CT. Trajectories of Lung Function in Infants and Children: Setting a Course for Lifelong Lung Health. Pediatrics 2020. Oct;146(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Martinez FD. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med 2016. Sep 1;375(9):871–8. [DOI] [PubMed] [Google Scholar]
- (5).Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med 2013. Nov;1(9):728–42. [DOI] [PubMed] [Google Scholar]
- (6).Morales E, Garcia-Esteban R, de la Cruz OA, Basterrechea M, Lertxundi A, de Dicastillo MD, et al. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax 2015. Jan;70(1):64–73. [DOI] [PubMed] [Google Scholar]
- (7).Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, et al. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med 2007. Aug 15;176(4):377–84. [DOI] [PubMed] [Google Scholar]
- (8).Lee AG, Kaali S, Quinn A, Delimini R, Burkart K, Opoku-Mensah J, et al. Prenatal Household Air Pollution Is Associated with Impaired Infant Lung Function with Sex-Specific Effects. Evidence from GRAPHS, a Cluster Randomized Cookstove Intervention Trial. Am J Respir Crit Care Med 2019. Mar 15;199(6):738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Spindel ER, McEvoy CT. The Role of Nicotine in the Effects of Maternal Smoking during Pregnancy on Lung Development and Childhood Respiratory Disease. Implications for Dangers of E-Cigarettes. Am J Respir Crit Care Med 2016. Mar 1;193(5):486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol 2002. Jan 1;26(1):31–41. [DOI] [PubMed] [Google Scholar]
- (11).Wongtrakool C, Roser-Page S, Rivera HN, Roman J. Nicotine alters lung branching morphogenesis through the alpha7 nicotinic acetylcholine receptor. Am J Physiol Lung Cell Mol Physiol 2007. Sep;293(3):L611–L618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Morrow LA, Wagner BD, Ingram DA, Poindexter BB, Schibler K, Cotten CM, et al. Antenatal Determinants of Bronchopulmonary Dysplasia and Late Respiratory Disease in Preterm Infants. Am J Respir Crit Care Med 2017. Aug 1;196(3):364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, et al. Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ 2013. Nov 8;62(6):1–19. [PubMed] [Google Scholar]
- (14).Filion KB, Abenhaim HA, Mottillo S, Joseph L, Gervais A, O’Loughlin J, et al. The effect of smoking cessation counselling in pregnant women: a meta-analysis of randomised controlled trials. BJOG 2011. Nov;118(12):1422–8. [DOI] [PubMed] [Google Scholar]
- (15).Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. ARRD 1992. May;145(5):1129–35. [DOI] [PubMed] [Google Scholar]
- (16).Li YF, Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Rappaport EB, et al. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med 2000. Dec;162(6):2097–104. [DOI] [PubMed] [Google Scholar]
- (17).Lodrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J 1997. Aug;10(8):1774–9. [DOI] [PubMed] [Google Scholar]
- (18).Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet 1996. Oct 19;348(9034):1060–4. [DOI] [PubMed] [Google Scholar]
- (19).Tager IB, Weiss ST, Munoz A, Rosner B, Speizer FE. Longitudinal study of the effects of maternal smoking on pulmonary function in children. nejm 1983. Sep 22;309(12):699–703. [DOI] [PubMed] [Google Scholar]
- (20).Hoo AF, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med 1998. Sep;158(3):700–5. [DOI] [PubMed] [Google Scholar]
- (21).Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA, Antova T, Gehring U, et al. Parental Smoking and Lung Function in Children: an International Study. Am J Respir Crit Care Med 2006. Feb 16. [DOI] [PubMed] [Google Scholar]
- (22).Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, O’Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: A prospective study. Thorax 2009. Jun 11;64(9):810–4. [DOI] [PubMed] [Google Scholar]
- (23).McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA 2014. May;311(20):2074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Young S, Le Souef PN, Geelhoed GC, Stick SM, Turner KJ, Landau LI. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med 1991. Apr 25;324(17):1168–73. [DOI] [PubMed] [Google Scholar]
- (25).McEvoy CT, Schilling D, Go MD, Mehess S, Durand M. Pulmonary function in extremely low birth weight infants with bronchopulmonary dysplasia before hospital discharge. J Perinatol 2021. Jan;41(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).McEvoy CT, Aschner JL. The Natural History of Bronchopulmonary Dysplasia: The Case for Primary Prevention. Clin Perinatol 2015. Dec;42(4):911–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Taglauer E, Abman SH, Keller RL. Recent advances in antenatal factors predisposing to bronchopulmonary dysplasia. Semin Perinatol 2018. Nov;42(7):413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Shepherd EG, Clouse BJ, Hasenstab KA, Sitaram S, Malleske DT, Nelin LD, et al. Infant Pulmonary Function Testing and Phenotypes in Severe Bronchopulmonary Dysplasia. Pediatrics 2018. May;141(5). [DOI] [PubMed] [Google Scholar]
- (29).Moschino L, Stocchero M, Filippone M, Carraro S, Baraldi E. Longitudinal Assessment of Lung Function in Survivors of Bronchopulmonary Dysplasia from Birth to Adulthood. The Padova BPD Study. Am J Respir Crit Care Med 2018. Jul 1;198(1):134–7. [DOI] [PubMed] [Google Scholar]
- (30).Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 2013. Aug;68(8):767–76. [DOI] [PubMed] [Google Scholar]
- (31).Fakhoury KF, Sellers C, Smith EO, Rama JA, Fan LL. Serial measurements of lung function in a cohort of young children with bronchopulmonary dysplasia. Pediatrics 2010. Jun;125(6):e1441–e1447. [DOI] [PubMed] [Google Scholar]
- (32).Doyle LW, Carse E, Adams AM, Ranganathan S, Opie G, Cheong JLY. Ventilation in Extremely Preterm Infants and Respiratory Function at 8 Years. N Engl J Med 2017. Jul 27;377(4):329–37. [DOI] [PubMed] [Google Scholar]
- (33).McEvoy C, Venigalla S, Schilling D, Clay N, Spitale P, Nguyen T. Respiratory function in healthy late preterm infants delivered at 33–36 weeks of gestation. J Pediatr 2013. Mar;162(3):464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kotecha SJ, Watkins WJ, Lowe J, Henderson AJ, Kotecha S. Effect of early-term birth on respiratory symptoms and lung function in childhood and adolescence. Pediatr Pulmonol 2016. Nov;51(11):1212–21. [DOI] [PubMed] [Google Scholar]
- (35).Haland G, Carlsen KC, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med 2006. Oct 19;355(16):1682–9. [DOI] [PubMed] [Google Scholar]
- (36).Guerra S, Lombardi E, Stern DA, Sherrill DL, Gilbertson-Dahdal D, Wheatley-Guy CM, et al. Fetal Origins of Asthma: A Longitudinal Study from Birth to Age 36 Years. Am J Respir Crit Care Med 2020. Dec 15;202(12):1646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).den Dekker HT, Jaddoe VWV, Reiss IK, de Jongste JC, Duijts L. Fetal and Infant Growth Patterns and Risk of Lower Lung Function and Asthma. The Generation R Study. Am J Respir Crit Care Med 2018. Jan 15;197(2):183–92. [DOI] [PubMed] [Google Scholar]
- (38).Ortqvist AK, Ullemar V, Lundholm C, Kuja-Halkola R, Magnusson PKE, Lichtenstein P, et al. Fetal Growth and Childhood Lung Function in the Swedish Twin Study on Prediction and Prevention of Asthma. Ann Am Thorac Soc 2017. Jul;14(7):1147–53. [DOI] [PubMed] [Google Scholar]
- (39).MacDonald KD, Vesco KK, Funk KL, Donovan J, Nguyen T, Chen Z, et al. Maternal body mass index before pregnancy is associated with increased bronchodilator dispensing in early childhood: A cross-sectional study. Pediatr Pulmonol 2016. Aug;51(8):803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Kallpur SG KS. Perinatal Modifiers of Lung Structure and Function. In: Jobe AHWJAS, editor. Fetal & neonatal lung development.New York: Cambridge University Press; 2016. p. 187–204. [Google Scholar]
- (41).Checkley W, West KP Jr., Wise RA, Baldwin MR, Wu L, LeClerq SC, et al. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med 2010. May 13;362(19):1784–94. [DOI] [PubMed] [Google Scholar]
- (42).McEvoy CT, Shorey-Kendrick LE, Milner K, Schilling D, Tiller C, Vuylsteke B, et al. Oral Vitamin C (500 mg/d) to Pregnant Smokers Improves Infant Airway Function at 3 Months (VCSIP). A Randomized Trial. Am J Respir Crit Care Med 2019. May 1;199(9):1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).McEvoy CT, Shorey-Kendrick LE, Milner K, Schilling D, Tiller C, Vuylsteke B, et al. Vitamin C to Pregnant Smokers Persistently Improves Infant Airway Function to 12 Months of Age: A Randomised Trial. Eur Respir J 2020. Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).McEvoy CT, Shorey-Kendrick LE, Milner K, Harris J, Vuylsteke B, Cunningham M, et al. Vitamin C supplementation (500 mg/day) for pregnant smokers and airway function and wheeze in their offspring at five years of age: follow-up of a randomized controlled trial. JAMA Pediatr 2023;177(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kole TM, Vanden Berghe E, Kraft M, Vonk JM, Nawijn MC, Siddiqui S, et al. Predictors and associations of the persistent airflow limitation phenotype in asthma: a post-hoc analysis of the ATLANTIS study. Lancet Respir Med 2022. Jul 27. [DOI] [PubMed] [Google Scholar]
- (46).Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med 2005. Nov 15;172(10):1253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol 2006. Nov;118(5):1040–7. [DOI] [PubMed] [Google Scholar]
- (48).Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J 2007. Sep;30(3):452–6. [DOI] [PubMed] [Google Scholar]
- (49).Tang M, Elicker BM, Henry T, Gierada DS, Schiebler ML, Huang BK, et al. Mucus Plugs Persist in Asthma, and Changes in Mucus Plugs Associate with Changes in Airflow over Time. Am J Respir Crit Care Med 2022. May 1;205(9):1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ 1996. May 11;312(7040):1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med 1988. Oct 27;319(17):1112–7. [DOI] [PubMed] [Google Scholar]
- (52).Morris MJ, Lane DJ. Tidal expiratory flow patterns in airflow obstruction. Thorax 1981. Feb;36(2):135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Hallas HW, Chawes BL, Arianto L, Rasmussen MA, Kunoe A, Stokholm J, et al. Children with Asthma Have Fixed Airway Obstruction through Childhood Unaffected by Exacerbations. J Allergy Clin Immunol Pract 2020. Apr;8(4):1263–71. [DOI] [PubMed] [Google Scholar]
- (54).Owens L, Laing IA, Zhang G, Turner S, Le Souef PN. Airway function in infancy is linked to airflow measurements and respiratory symptoms from childhood into adulthood. Pediatr Pulmonol 2018. Aug;53(8):1082–8. [DOI] [PubMed] [Google Scholar]
- (55).Malmberg LP, Seppa VP, Kotaniemi-Syrjanen A, Malmstrom K, Kajosaari M, Pelkonen AS, et al. Measurement of tidal breathing flows in infants using impedance pneumography. Eur Respir J 2017. Feb;49(2). [DOI] [PubMed] [Google Scholar]
- (56).Chawes BL, Stokholm J, Bonnelykke K, Brix S, Bisgaard H. Neonates with reduced neonatal lung function have systemic low-grade inflammation. J Allergy Clin Immunol 2015. Jun;135(6):1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Kotaniemi-Syrjanen A, Malmberg LP, Pelkonen AS, Malmstrom K, Makela MJ. Airway responsiveness: associated features in infants with recurrent respiratory symptoms. Eur Respir J 2007. Dec;30(6):1150–7. [DOI] [PubMed] [Google Scholar]
- (58).Jackson CM, Mukherjee S, Wilburn AN, Cates C, Lewkowich IP, Deshmukh H, et al. Pulmonary Consequences of Prenatal Inflammatory Exposures: Clinical Perspective and Review of Basic Immunological Mechanisms. Front Immunol 2020;11:1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Kunzmann S, Collins JJ, Kuypers E, Kramer BW. Thrown off balance: the effect of antenatal inflammation on the developing lung and immune system. Am J Obstet Gynecol 2013. Jun;208(6):429–37. [DOI] [PubMed] [Google Scholar]
- (60).Strang LB. Eosinophilia in children with asthma and bronchiectasis. Br Med J 1960. Jan 16;1(5167):167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Hogarth-Scott RS, Howlett BJ, McNicol KN, Simons MJ, Williams HE. IgE levels in the sera of asthmatic children. Clin Exp Immunol 1971. Nov;9(5):571–6. [PMC free article] [PubMed] [Google Scholar]
- (62).Khoo SK, Read J, Franks K, Zhang G, Bizzintino J, Coleman L, et al. Upper Airway Cell Transcriptomics Identify a Major New Immunological Phenotype with Strong Clinical Correlates in Young Children with Acute Wheezing. J Immunol 2019. Mar 15;202(6):1845–58. [DOI] [PubMed] [Google Scholar]
- (63).Achten NB, van Rossum AMC, Bacharier LB, Fitzpatrick AM, Hartert TV. Long-Term Respiratory Consequences of Early-Life Respiratory Viral Infections: A Pragmatic Approach to Fundamental Questions. J Allergy Clin Immunol Pract 2022. Mar;10(3):664–70. [DOI] [PubMed] [Google Scholar]
- (64).Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A 2008. Sep 9;105(36):13562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Oo SWC, Khoo SK, Cox DW, Chidlow G, Franks K, Prastanti F, et al. Defining Age-specific Relationships of Respiratory Syncytial Virus and Rhinovirus Species in Hospitalized Children With Acute Wheeze. Pediatr Infect Dis J 2021. Oct 1;40(10):873–9. [DOI] [PubMed] [Google Scholar]
- (66).Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011. Mar 17;364(11):1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med 2012. May 4;18(5):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008. Oct 1;178(7):667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Makrinioti H, Hasegawa K, Lakoumentas J, Xepapadaki P, Tsolia M, Castro-Rodriguez JA, et al. The role of respiratory syncytial virus- and rhinovirus-induced bronchiolitis in recurrent wheeze and asthma-A systematic review and meta-analysis. Pediatr Allergy Immunol 2022. Mar;33(3):e13741. [DOI] [PubMed] [Google Scholar]
- (70).Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol 2007. May;119(5):1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol 2014. Aug;134(2):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Chirkova T, Rosas-Salazar C, Gebretsadik T, Jadhao SJ, Chappell JD, Peebles RS Jr., et al. Effect of Infant RSV Infection on Memory T Cell Responses at Age 2–3 Years. Front Immunol 2022;13:826666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007. Oct 11;357(15):1487–95. [DOI] [PubMed] [Google Scholar]
- (74).Holt PG. The mechanism or mechanisms driving atopic asthma initiation: The infant respiratory microbiome moves to center stage. J Allergy Clin Immunol 2015. Jul;136(1):15–22. [DOI] [PubMed] [Google Scholar]
- (75).Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015. May 13;17(5):704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA 2015. Nov 17;314(19):2034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Cuthbertson L, Oo SWC, Cox MJ, Khoo SK, Cox DW, Chidlow G, et al. Viral respiratory infections and the oropharyngeal bacterial microbiota in acutely wheezing children. PLoS One 2019;14(10):e0223990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Jackson DJ, Gern JE. Rhinovirus Infections and Their Roles in Asthma: Etiology and Exacerbations. J Allergy Clin Immunol Pract 2022. Mar;10(3):673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 2010. Jan 19;18(1):8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Heijink IH, Kuchibhotla VNS, Roffel MP, Maes T, Knight DA, Sayers I, et al. Epithelial cell dysfunction, a major driver of asthma development. Allergy 2020. Aug;75(8):1902–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Kim S, Forno E, Yan Q, Jiang Y, Zhang R, Boutaoui N, et al. SNPs identified by GWAS affect asthma risk through DNA methylation and expression of cis-genes in airway epithelium. Eur Respir J 2020. Apr;55(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Brugha R, Lowe R, Henderson AJ, Holloway JW, Rakyan V, Wozniak E, et al. DNA methylation profiles between airway epithelium and proxy tissues in children. Acta Paediatr 2017. Dec;106(12):2011–6. [DOI] [PubMed] [Google Scholar]
- (83).Dai X, Dharmage SC, Lodge CJ. Interactions between glutathione S-transferase genes and household air pollution on asthma and lung function. Front Mol Biosci 2022;9:955193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Henderson AJ, Newson RB, Rose-Zerilli M, Ring SM, Holloway JW, Shaheen SO. Maternal Nrf2 and gluthathione-S-transferase polymorphisms do not modify associations of prenatal tobacco smoke exposure with asthma and lung function in school-aged children. Thorax 2010. Oct;65(10):897–902. [DOI] [PubMed] [Google Scholar]
- (85).Murdzoska J, Devadason SG, Khoo SK, Landau LI, Young S, Goldblatt J, et al. In utero smoke exposure and role of maternal and infant glutathione s-transferase genes on airway responsiveness and lung function in infancy. Am J Respir Crit Care Med 2010. Jan 1;181(1):64–71. [DOI] [PubMed] [Google Scholar]
- (86).Owens L, Laing IA, Murdzoska J, Zhang G, Turner SW, Le Souef PN. Glutathione S-Transferase Genotype Protects against In Utero Tobacco-linked Lung Function Deficits. Am J Respir Crit Care Med 2019. Aug 15;200(4):462–70. [DOI] [PubMed] [Google Scholar]
- (87).Turner S, Francis B, Wani N, Vijverberg S, Pino-Yanes M, Mukhopadhyay S, et al. Variants in genes coding for glutathione S-transferases and asthma outcomes in children. Pharmacogenomics 2018. Jun 1;19(8):707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Gilliland FD, Li YF, Dubeau L, Berhane K, Avol E, McConnell R, et al. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2002. Aug 15;166(4):457–63. [DOI] [PubMed] [Google Scholar]
- (89).Kabesch M, Hoefler C, Carr D, Leupold W, Weiland SK, von ME. Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax 2004. Jul;59(7):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


