Abstract
Severe traumatic brain injury (TBI) is often associated with diffuse axonal injury. Diffuse axonal injury affecting the corpus callosum may present with intraventricular hemorrhage on baseline computed tomography (CT) scan. Posttraumatic corpus callosum damage is a chronic condition that can be diagnosed over the long term using various magnetic resonance imaging (MRI) sequences. Here, we present two cases of severe survivors of TBI with isolated intraventricular hemorrhage detected on an initial CT scan. After acute trauma management, long-term follow-up was performed. Diffusion tensor imaging and subsequent tractography revealed a significant decrease in the fractional anisotropy values and the number of corpus callosum fibers compared with those in healthy control patients. This study presents a possible correlation between traumatic intraventricular hemorrhage on admission CT and long-term corpus callosum impairment detected on MRI in patients with severe head injury by presenting demonstrative cases and conducting a literature review.
Keywords: Traumatic brain injury, Diffuse axonal injury, Cerebral intraventricular hemorrhage, Corpus callosum, Magnetic resonance imaging, Diffusion tensor imaging
INTRODUCTION
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality among young individuals worldwide. The incidence of TBI in developed countries is estimated to be about 200 people per 100,000 population. According to the World Health Organization, TBI is the third most common cause of disability in the world3,18) TBI can present as mild, moderate, or severe according to the Glasgow Coma Scale (GCS) score.15) In about 50% of the cases with severe TBI, a diffuse axonal injury (DAI) is present. The most commonly affected brain areas by DAI are the brainstem, the corpus callosum (CC), the frontal, temporal, and parasagittal white matter (WM), the cerebral cortex, and the cerebellum.4,15)
Intraventricular hemorrhage (IVH) is a rare finding among TBI patients. According to the initial computed tomography (CT) scan, the IVH can be classified as isolated or associated with minor or significant CT findings.2) The severity of IVH is classified according to the original or the more specific modified Graeb scale.17,22) There are some studies that suggest the possible correlation between CC injury and traumatic IVH.6,8,12,13,14,21) The presence of CC injury and its long-term impairment can be detected by the use of the conventional T1 and T2 magnetic resonance imaging (MRI) sequences, as well as specific structural sequences such as FLAIR, susceptibility-weighted imaging (SWI), diffusion tensor imaging (DTI) and diffusion tensor tractography (DTT).16) DTI and DTT are useful tools for evaluating long-term WM changes in patients with TBI. DTI has multiple indexes for measuring WM integrity, but the most used among scientists is fractional anisotropy (FA). Values of FA closer to 0 correspond to WM impairment.20) Here we present two case reports of patients after severe TBI and isolated traumatic IVH on the initial CT with long-term CC impairment evaluated by advanced MRI sequences and 3D tensor tractography.
CASE REPORT
Patient description
Clinical and neuroimaging data of two adult male patients with severe TBI who were admitted to the intensive care unit (ICU) and Clinic of Neurosurgery of our institution are presented. Both patients had evidence of IVH on the initial CT scan. After the trauma management, a long-term follow-up of the patients was performed. During the follow-up we obtained a MRI, detailed neurological examination, and an evaluation of cognitive functions by applying the Mini-Mental State Examination (MMSE) scale where scores under 24 indicate cognitive compromise in educated individuals while scores under 10 indicate severe cognitive compromise. Disease outcome was assessed by the Extended Glasgow Outcome Scale (GOS-E) which provides detailed categorization of functional outcome into eight categories. The results were compared to two, compatible by age, sex, and education of healthy controls.
Neuroimaging data acquisition
A 3.0T scanner (GE Discovery 750w; GE HealthCare, Chicago, IL, USA) was used to collect the images. The MRI protocol consisted of a T1-weighted imaging, turbo spin echo T2-weighted imaging, axial T2-weighted gradient echo imaging, FLAIR, DWI, SWI, and DTI. The diffusion images were acquired using a 2D EPI diffusion sequence (TE=108.6 ms, and TR=9000 ms). A DTI diffusion scheme was used, and a total of 25 diffusion sampling directions were acquired. The b-value was 1000.46 s/mm2. The in-plane resolution was 1.0156 mm. The slice thickness was 4 mm. A licensed neuroradiologist interpreted and analyzed all MRI data.
DTI postprocessing and data analysis
Diffusion data was postprocessed using DSI Studio (http://dsi-studio.labsolver.org). The FA was optimized and thinned with a threshold of >0.2. An automated seeding region was placed at the CC. The anisotropy threshold was randomly selected. The angular threshold was randomly selected from 15 degrees to 90 degrees. The step size was randomly selected from 0.5 voxels to 1.5 voxels. Tracks with lengths shorter than 20 or longer than 300 mm were discarded. A total of 1000000 seeds were placed. Additional measured data included radial diffusion, axial diffusion, normalized quantitative anisotropy, tract volume, and number of fibers. The anatomy was extracted from a tractography atlas, to reconstruct a 3D model of the CC.25) We have used a two-tailed t-test to compare FA values and number of fibers between the patients and the control subjects. The bilateral significance level of both variables was determined to be p<0.05.
Case 1
A 17-year-old male patient was transferred to the emergency room presenting a GCS score of 5 points (E1V1M3) after a motor vehicle accident. The whole-body CT revealed evidence of thoracic and abdominal injuries, as well as isolated IVH in the occipital horn of the left lateral ventricle, corresponding with 1 point on the modified Graeb scale (FIGURE 1A). No radiological data of cervical trauma was found. The patient was treated conservatively in the ICU and the Clinic of Neurosurgery. He regained consciousness 14 days following the accident but remained unresponsive and with persistent quadriparesis. At discharge, the patient was evaluated as Grade 3 according to the GOS-E, which is considered an unfavorable outcome from TBI.
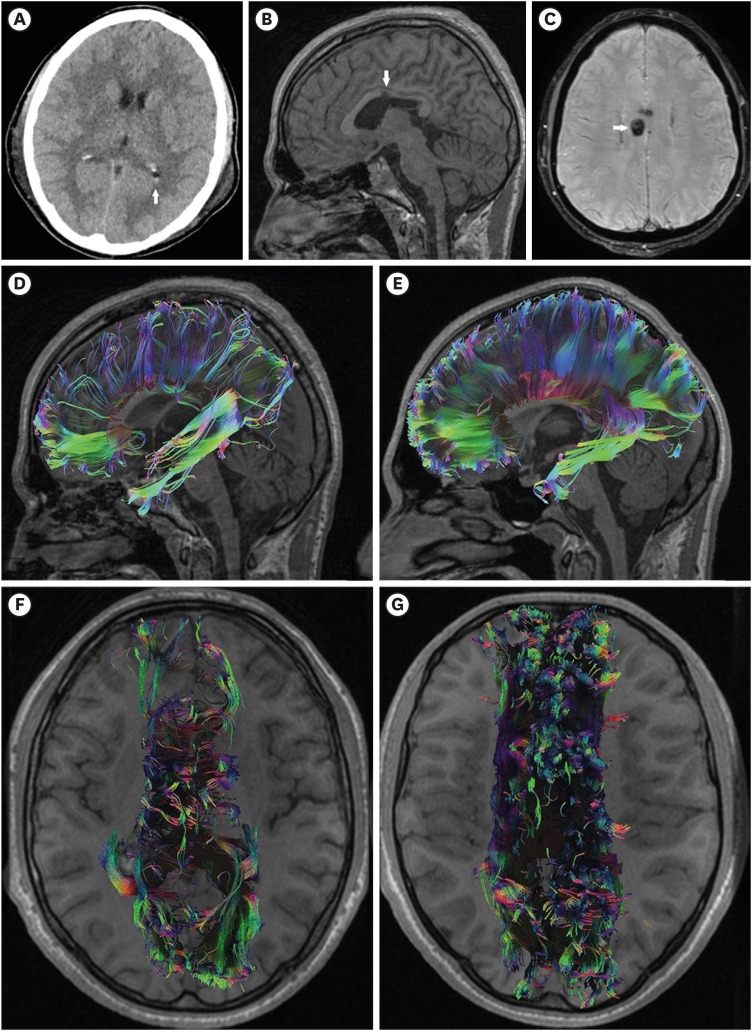
FIGURE 1. Case 1. (A) Admission computed tomography scan showing the presence of isolated intraventricular hemorrhage in the occipital horn of the left lateral ventricle (arrow); (B-G) A 13-months follow-up magnetic resonance imaging is demonstrated; (B) Sagittal T1 shows disruption of the body of the corpus callosum (arrow); (C) susceptibility-weighted imaging shows hemosiderin deposits in the body of the corpus callosum (arrow); (D) Sagittal corpus callosum tractography demonstrates a decrease in the number of fibers as well as distortion of the anatomy in comparison to a reconstruction of a control subject (E); (F) Axial corpus callosum tractography demonstrated bilateral corpus callosum impairment in comparison to a control subject (G).
The follow-up was performed 13 months after the trauma. The patient was adequate and responsive, but hypobulic and emotionally labile. Detailed neurological examination revealed residual right hemiparesis. The cognitive functions were examined using the MMSE scale and mild cognitive impairments were established (23/30p.), affecting the attention and memory cognitive domains. The patient was classified as Grade 4 according to GOS-E. On MRI, the sagittal T1 and the SWI demonstrated chronic posttraumatic impairments of the brain, located mostly in the body of the CC (FIGURE 1B & C). DTI and DTT revealed a decrease in the FA value and in the fibers of the CC bilaterally, compared to a healthy control subject (FIGURE 1D-G, TABLE 1).
TABLE 1. Comparison between FA values and number of fibers of corpus callosum of the two cases and two control subjects.
| Variables | FA | Number of fibers |
|---|---|---|
| Cases | 0.434±0.01 | 4,996±764 |
| Control subjects | 0.507±0.004 | 83,542±1,243 |
| p-value | 0.005 | 0.003 |
Values are presented as mean ± standard deviation.
FA: fractional anisotropy.
Case 2
A 52-year-old male patient was transferred to the emergency room presenting a GCS of 7 points (E2V2M3) after a fall from his height. The whole-body CT scan revealed bilateral IVH in the occipital horns of the lateral ventricles. No radiological evidence of cervical, thoracic, or abdominal trauma was found. The patient was assessed with 2 points on the modified Graeb scale (FIGURE 2A). The subsequent CT scan demonstrated a contusion of the right cerebellar hemisphere. The patient was treated conservatively in the ICU and the Clinic of Neurosurgery. He regained consciousness four days following the accident. At discharge, the patient was evaluated as Grade 4 according to GOS-E, which is considered an unfavorable outcome of TBI.
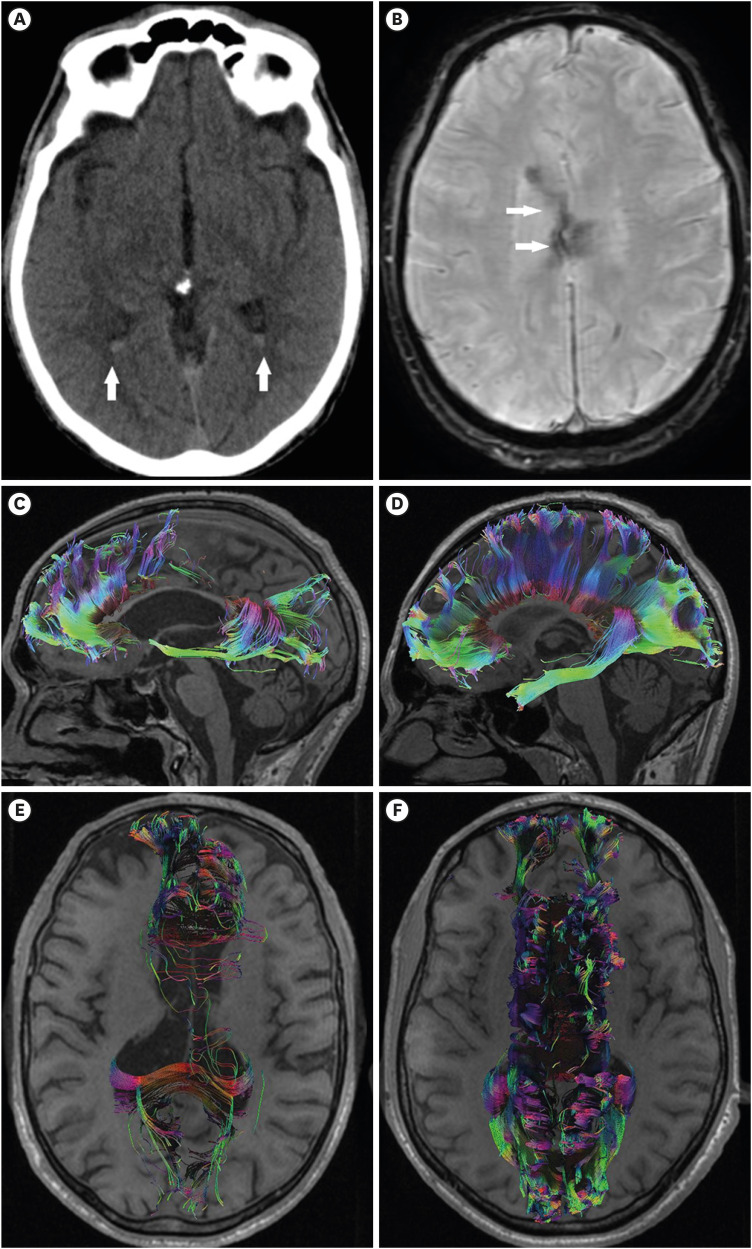
FIGURE 2. Case 2. (A) The admission computed tomography scan shows the presence of isolated intraventricular hemorrhage located in the occipital horns of the lateral ventricles (arrows). (B, C) A 50-month follow-up magnetic resonance imaging is shown. (B) The susceptibility-weighted imaging demonstrates hemosiderin deposits in the body of the corpus callosum (arrows). (C) Corpus callosum tractography shows a significantly decreased number of fibers, especially in the body of the corpus callosum, compared to healthy control (D). (E) Axial corpus callosum tractography demonstrated bilateral corpus callosum impairment in comparison to a control subject (F).
The follow-up was performed 50 months after the trauma. Detailed neurological examination detected diverse persistent focal neurological symptoms such as ataxia, dyscoordination, dysphagia, dysarthria, dysphonia, and residual left hemiparesis. The cognitive functions were examined using the MMSE scale and mild cognitive impairments were established (23/30p.), affecting the attention, memory, and concentration cognitive domains. According to GOS-E the patient was classified as Grade 6. The SWI presented with chronic posttraumatic hemosiderosis, located mostly in the body of the CC (FIGURE 2B). DTI and DTT showed a decrease in the value of FA and in the number of fibers of the CC bilaterally (FIGURE 2C-F, TABLE 1).
DISCUSSION
The most common causes of TBI are high-velocity motor vehicle accidents, falls from heights, assaults, and sports injuries.1,5) TBI can present with soft tissue head injuries, skull fractures, intracranial hematomas (epidural, subdural, and intracerebral), cerebral contusions, subarachnoid and IVH, as well as DAI.9) In about 50% of the cases with severe TBI, DAI is present, because of stretching, torsion, and multiple ruptures of axons at the transition between the gray and white brain matter, due to a sudden acceleration-deceleration or rotation of the head and brain. The most affected brain areas by DAI are the brainstem, CC, frontal, temporal, and parasagittal WM, the cerebral cortex, and the cerebellum.4,15) The same mechanism of injury may cause disruption of subependymal brain vessels located in the septum pellucidum, fornix, or anterior commissure, which results in traumatic IVH.21)
IVH is an uncommon finding in patients with TBI. The incidence of IVH is reported by different studies to be 0.4%–22%, with higher rates of occurrence in patients with severe TBI.11,14,19,21) IVH can be classified as isolated or associated with minor or significant CT findings. An isolated traumatic IVH correlates with no other findings on initial head CT, except for a contusion in the basal ganglia.2) In both our cases there was evidence of isolated IVH on the initial CT following the accident. The severity of IVH is classified according to the Graeb scale. The original Graeb scale is based on whether the third, fourth, right, and/or left lateral ventricles are expanded and filled with blood. A maximum score of 4 is given for each lateral ventricle and a maximum score of 2 is given for the third and fourth ventricles with a maximum possible score of 12.22) Later, the more specific modified Graeb score was developed by dividing the ventricles into compartments with a maximum possible score of 32 in which every compartment is filled with blood and expanded.17) The IVH of our cases was classified according to the modified Graeb scale due to its detailed division of the ventricles.
The appearance of traumatic IVH on the initial CT is often associated with increased mortality and morbidity, poor outcome, and long-term prognosis. According to several studies, the mortality rate of IVH in patients after TBI varies from 11.4% to 65%.2,11,19,21) In the presented cases, the patients suffered from severe TBI with poor outcomes. There is a limited number of studies concerning the association between CC injury on MRI and the presence of traumatic IVH on CT6,8,12,13,14) (TABLE 2). The CC connects the left and right cerebral hemispheres and represents the largest brain commissure maintaining cognitive function, working memory, bimanual coordination, and motor function. It is one of the most vulnerable neural structures in the brain susceptible to DAI due to its midline location.5,10,13) The diagnosis of DAI is based on the data from the clinical examination and contemporary neuroimaging studies obtained after the traumatic event. The clinical symptoms may vary from mild to severe but, usually, these are patients with severe TBI with a score ≤8 points according to the GCS with a duration of at least 6 hours.24) Furthermore, the diagnosis is confirmed by means of neuroimaging.
TABLE 2. Overview of published cases concerning the association between CC injury on MRI and the presence of traumatic IVH on baseline CT.
| Authors | No. of reported cases | Admission CT hemorrhagic findings | Time to MRI follow-up | MRI findings |
|---|---|---|---|---|
| Gadda et al., 20046) | 1 | IVH in the third ventricle and fornix; Subcortical fronto-temporal hemorrhage; Parieto-occipital subarachnoid hemorrhage; Left thalamic haemorrhage | 21 days | Lesions located in the body and splenium of CC |
| Gentry et al., 19888) | 9 | IVH limited to the occipital horns; In one case IVH is present in the third ventricle | 2–19 days | Nonhemorrhagic DAI located in the splenium of the CC |
| Lolli et al., 201612) | 1 | IVH in the occipital horn of the right lateral ventricle combined with subdural hematoma | 24–72 hours | DAI located in the splenium of the CC |
| Mata-Mbemba et al., 201513) | 13 | IVH combined with subarachnoid hemorrhage; In one case isolated IVH | 0–30 days | CC injury, combined with lesion in the lobar white matter, cerebellum or brainstem |
| Matsukawa et al., 201214) | 11 | IVH | 14 days | CC injury |
| Our study, 2023 | 2 | IVH | 13–50 months | Injury of the body of CC |
CC: corpus callosum, MRI: magnetic resonance imaging, IVH: intraventricular hemorrhage, CT: computed tomography, DAI: diffuse axonal injury.
The CT examination is still the gold standard for imaging of TBI in terms of emergency due to its speed, accessibility, and accuracy in detecting traumatic intracranial hemorrhages and cranial fractures.23) CC injury on CT in the acute phase may present as a hyperdense hemorrhagic lesion in size from a few millimeters to a few centimeters in diameter.7) In some cases, TBI and CC injury is difficult to diagnose due to the lack of sufficient radiological evidence on the performed CT scans.7) This has led to the increased use of other more sensitive methods for neuroimaging, namely various MRI sequences such as FLAIR, SWI, DTI, and resting state MRI.7,16,20) The T1, and T2, as well as the FLAIR, may be useful to examine the macroscopic structural lesions and contusions following TBI,20) as presented in Case 1 (FIGURE 1). On the other hand, SWI employs the magnetic properties of iron for detecting microscopic bleeds and persistent hemosiderin deposits, particularly for DAI in the cerebral gray-WM junction areas, CC, the subcortical WM, brainstem and intraventricular and subarachnoid hemorrhage.7) In our cases, SWI established long-term hemosiderin deposits, located mainly in the body of the CC.
On the other side, DTI is an advanced MRI sequence, able to sensitively detect microstructural WM changes in patients with TBI. DTI evaluates WM impairment by measuring the direction of diffusion of water molecules along WM fibers.16) DTI has multiple indexes for measuring WM integrity, but the most used among scientists is FA, which describes the direction of water molecules. FA is a measure, that range from 0 to 1. FA measured closer to 0 corresponds to WM impairment. FA is a sensitive marker for detecting changes in the CC integrity in patients with TBI.4,20) The presented cases demonstrated a reduction of FA values of the CC in both patients, compared to healthy control individuals (p=0.005). Additionally, DTI can also be post-processed for creating tractography of different fiber tracts, commonly affected by TBI, such as CC.20)
As presented in our cases, the initial CT showed only the presence of IVH and no information about direct CC injury. However, the various MRI modalities demonstrated long-term CC injury in both patients. To the best of our knowledge, this is the first paper that demonstrates a correlation between isolated IVH on baseline CT and long-term changes of CC detected by means of DTT and DTI. The major limitation of this study is the insufficient number of participants due to the rarity of the isolated IVH and its common association with other intracranial traumatic lesions. Further studies including a larger number of patients are needed to elucidate the association between isolated IVH and long-term impairment of CC after sustained severe TBI, as well as the potential role of isolated IVH as a prognostic biomarker of CC patency in relationship to the neurological outcomes of these patients.
CONCLUSION
Isolated traumatic IVH is a rare entity among patients with severe TBI. According to the presented cases and the conducted literature review, we suggest that the presence of isolated traumatic IVH on the initial CT may be related to a long-term CC impairment. CC injury is an important condition that is difficult to diagnose and may lead to chronic physical and cognitive impairment in patients who sustained severe TBI.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Aoun R, Rawal H, Attarian H, Sahni A. Impact of traumatic brain injury on sleep: an overview. Nat Sci Sleep. 2019;11:131–140. doi: 10.2147/NSS.S182158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atzema C, Mower WR, Hoffman JR, Holmes JF, Killian AJ, Wolfson AB, et al. Prevalence and prognosis of traumatic intraventricular hemorrhage in patients with blunt head trauma. J Trauma. 2006;60:1010–1017. doi: 10.1097/01.ta.0000218038.28064.9d. [DOI] [PubMed] [Google Scholar]
- 3.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44:2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang MC, Jang SH. Corpus callosum injury in patients with diffuse axonal injury: a diffusion tensor imaging study. NeuroRehabilitation. 2010;26:339–345. doi: 10.3233/NRE-2010-0571. [DOI] [PubMed] [Google Scholar]
- 5.Frati A, Cerretani D, Fiaschi AI, Frati P, Gatto V, La Russa R, et al. Diffuse axonal injury and oxidative stress: a comprehensive review. Int J Mol Sci. 2017;18:2600. doi: 10.3390/ijms18122600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadda D, Carmignani L, Vannucchi L, Bindi A. Traumatic lesions of corpus callosum: early multidetector CT findings. Neuroradiology. 2004;46:812–816. doi: 10.1007/s00234-004-1250-y. [DOI] [PubMed] [Google Scholar]
- 7.Gaillard F, Sharma R, Micał W, et al. Diffuse axonal injury. Melbourne, Victoria: Radiopaedia; 2013. [Accessed December 16, 2022]. https://radiopaedia.org/articles/13562 . [Google Scholar]
- 8.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol. 1988;150:663–672. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- 9.Hawryluk GW, Manley GT. Classification of traumatic brain injury: past, present, and future. Handb Clin Neurol. 2015;127:15–21. doi: 10.1016/B978-0-444-52892-6.00002-7. [DOI] [PubMed] [Google Scholar]
- 10.Jang SH, Kim OL, Kim SH, Lee HD. Differences in corpus callosum injury between cerebral concussion and diffuse axonal injury. Medicine (Baltimore) 2019;98:e17467. doi: 10.1097/MD.0000000000017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CY, Chuang CC, Chen CC, Tu PH, Wang YC, Yeap MC, et al. The role of intraventricular hemorrhage in traumatic brain injury: a novel scoring system. J Clin Med. 2022;11:2127. doi: 10.3390/jcm11082127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lolli V, Pezzullo M, Delpierre I, Sadeghi N. MDCT imaging of traumatic brain injury. Br J Radiol. 2016;89:20150849. doi: 10.1259/bjr.20150849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mata-Mbemba D, Mugikura S, Nakagawa A, Murata T, Kato Y, Tatewaki Y, et al. Intraventricular hemorrhage on initial computed tomography as marker of diffuse axonal injury after traumatic brain injury. J Neurotrauma. 2015;32:359–365. doi: 10.1089/neu.2014.3453. [DOI] [PubMed] [Google Scholar]
- 14.Matsukawa H, Shinoda M, Fujii M, Takahashi O, Murakata A, Yamamoto D, et al. Intraventricular hemorrhage on computed tomography and corpus callosum injury on magnetic resonance imaging in patients with isolated blunt traumatic brain injury. J Neurosurg. 2012;117:334–339. doi: 10.3171/2012.5.JNS112318. [DOI] [PubMed] [Google Scholar]
- 15.Mesfin FB, Gupta N, Hays Shapshak A, Taylor RS. Diffuse axonal injury. Tampa, FL: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 16.Michel BF, Sambuchi N, Vogt BA. Impact of mild traumatic brain injury on cingulate functions. Handb Clin Neurol. 2019;166:151–162. doi: 10.1016/B978-0-444-64196-0.00010-8. [DOI] [PubMed] [Google Scholar]
- 17.Morgan TC, Dawson J, Spengler D, Lees KR, Aldrich C, Mishra NK, et al. The Modified Graeb Score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke. 2013;44:635–641. doi: 10.1161/STROKEAHA.112.670653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popescu C, Anghelescu A, Daia C, Onose G. Actual data on epidemiological evolution and prevention endeavours regarding traumatic brain injury. J Med Life. 2015;8:272–277. [PMC free article] [PubMed] [Google Scholar]
- 19.Ravi K, Reddy MS, Gollapudi PR, Mohammed I, Manne S, Beniwal HK. Posttraumatic isolated intraventricular hemorrhage a rare entity: case series. Asian J Neurosurg. 2019;14:162–165. doi: 10.4103/ajns.AJNS_171_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibahashi K, Sugiyama K, Okura Y, Hoda H, Hamabe Y. Intraventricular hemorrhage after head injury: a multicenter, retrospective, cohort study. World Neurosurg. 2018;114:e350–e355. doi: 10.1016/j.wneu.2018.02.183. [DOI] [PubMed] [Google Scholar]
- 22.Trifan G, Arshi B, Testai FD. Intraventricular hemorrhage severity as a predictor of outcome in intracerebral hemorrhage. Front Neurol. 2019;10:217. doi: 10.3389/fneur.2019.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsitsopoulos PP, Abu Hamdeh S, Marklund N. Current opportunities for clinical monitoring of axonal pathology in traumatic brain injury. Front Neurol. 2017;8:599. doi: 10.3389/fneur.2017.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira RC, Paiva WS, de Oliveira DV, Teixeira MJ, de Andrade AF, de Sousa RM. Diffuse axonal injury: epidemiology, outcome and associated risk factors. Front Neurol. 2016;7:178. doi: 10.3389/fneur.2016.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh FC, Panesar S, Fernandes D, Meola A, Yoshino M, Fernandez-Miranda JC, et al. Population-averaged atlas of the macroscale human structural connectome and its network topology. Neuroimage. 2018;178:57–68. doi: 10.1016/j.neuroimage.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]