Abstract
Multiple sclerosis (MS) is a central nervous system (CNS) demyelinating disease. Failure to successfully remyelinate is common in MS lesions, often with consequent neuronal/axonal damage. CNS myelin is normally made by oligodendroglial cells (OLs). Remyelination by Schwann cells (SchC) has been reported in spinal cord demyelination, where SchCs are in close proximity to CNS myelin. We identified an MS cerebral lesion that was remyelinated by SchCs. This prompted us to query the extent of SchC remyelination in the brains and spinal cords of additional autopsied MS specimens.
CNS tissues were obtained from the autopsies of 14 MS cases. Remyelinated lesions were identified by LFB-PAS and solochrome cyanine stainings. Deparaffinized sections containing remyelinated lesions were stained with anti-GFAP to identify reactive astrocytes. P0 is a protein exclusive to peripheral but not CNS myelin. Areas of SchC remyelination were identified by staining with anti-P0. Proteolipid protein (PLP) is Myelinated regions in the index case cerebral lesion were confirmed to be of SchC origin using anti-P0 staining. Subsequently, 64 MS lesions from 14 autopsied MS cases were examined, and 23 lesions in 6 cases showed remyelination by SchCs. Lesions from the cerebrum, brainstem, and spinal cord were examined in each case. When present, SchC remyelination was most commonly located adjacent to venules and was associated with a lower surrounding density of GFAP+ reactive astrocytes than areas of only OL remyelination. The difference was significant only for spinal cord and brainstem lesions but not for lesions located in the brain.
In conclusion, we demonstrated SchC remyelination in the cerebrum, brainstem, and spinal cord of 6 autopsied MS cases. To our knowledge, this is the first report of supratentorial SchC remyelination in MS.
Introduction
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS) characterized by neuroinflammation, demyelination, and axonal damage. In MS, demyelination is the result of an immune-mediated insult targeting oligodendrocytes and the myelin produced by them1. Remyelination, the process in which myelin sheaths are restored to demyelinated axons, can be extensive in people with MS (pwMS) and effective at reinstating saltatory conduction2. However, remyelination is variable; failure to successfully remyelinate is frequently reported in longstanding or progressive MS3,4. Remyelination failure likely leads to axonal and neuronal loss1. In most circumstances, the new myelinforming cells in MS are oligodendrocytes (OLs). However, in MS and its experimental models, CNS spinal cord remyelination can be mediated by Schwann cells5. Schwann cells (SchCs) share with OLs the function of myelination of axons, but in the peripheral nervous system (PNS). Despite this commonality of function, SchCs and OLs are anatomically and evolutionarily distinct, producing myelin of different protein and lipid compositions. Both PNS and CNS myelin contain myelin basic protein (MBP)6. However, the glycoprotein P zero (P0) is unique to PNS myelin produced by SchCs, while proteolipid protein (PLP) is unique to CNS myelin6. When using the Luxol fast blue PAS (LFB-PAS) stain, myelin produced by SchCs has a slightly different color from that made by OLs7. This creates a visual delineation of most cranial nerves and spinal nerve roots at the point where PNS myelin switches to CNS myelin8. Animal studies demonstrated SchCs participation in the remyelination of demyelinated axons in the spinal cord, several decades ago9. However, SchC-derived remyelination has been infrequently noted in humans, and when it has, it has been reported primarily in the spinal cord5. This study investigated remyelination by SchC in post-mortem CNS tissues from pwMS. We describe the presence of SchCs in demyelinating cerebral and spinal cord MS plaques, confirmed by immunohistochemical staining for P0 protein. In our samples, SchC remyelination adjacent to venules was common. SchC remyelination was associated with fewer GFAP+ reactive astrocytes in the lesion.
Materials and Methods
Index case
CNS tissues from the autopsy of a 33-year-old male with an 11-year history of relapsing-remitting multiple sclerosis (RRMS), who died from hypertrophic cardiomyopathy, were examined by a neuropathologist (RES) and noted to have remyelination of a distinct color from CNS myelin, suggestive of SchC remyelination. The remyelinated area was deep within a cerebral MS lesion.
Human CNS Tissues
Additional CNS tissues were obtained from The Neuroimmunology Section Tissue Repository at Washington University in St. Louis. Specimens consisted of cerebrum, brainstem, and spinal cord and were obtained from an additional 13 patients diagnosed during life with MS. A total of 64 lesions from 14 autopsies were analyzed, including the index case. Demographic and clinical information can be found in Table 1.
Table 1.
Demographic and clinic characteristic of the 14 autopsied cases.
| Case | Age/Gender | Disease duratio n to death | Cause of death | MS Type | Disease Modifying Therapies | EDSS (at the time of death) | Brain (P0 myelin quantification) | Brainstem (P0 myelin quantification) | Spinal cord-anterior (P0 myelin quantification) | Spinal cord – posterior (P0 myelin quantification) |
|---|---|---|---|---|---|---|---|---|---|---|
| Index case | ||||||||||
| 1 | 33/M | 11 years | hypertrophic cardiomyopathy | RRMS | Interferon-β 1b | 2.5 | +++ | ++ (PONS) | ++ (TSC) | +++ (TSC) |
| MS cases with P0+ Immunostaining | ||||||||||
| 2 | 64/M | 18 years | metastatic melanoma | SPMS | None | 7.0 | + | Ø | +++ (TSC) +++ (CSC) |
Ø |
| 3 | 54/F | 17 years | pneumonia | SPMS | Interferon-β 1b | 7.0 | Ø | + (MED) | ++(CSC) | Ø |
| 4 | 32/F | 6 years | pneumonia | RRMS | Unknown | Unknown | + | + (PONS) | ++ (TSC) | Ø |
| 5 | 54/F | 22 years | pneumonia | SPMS | None | 9.0 | Ø | Ø | Ø | ++ (CSC) |
| 6 | 45/F | 7 years | respiratory failure | SPMS | None | 9.0 | Ø | Ø | +++ (CSC) | +++ (CSC) |
| MS Cases without P0+ Immunostaining | ||||||||||
| 7 | 41/F | 15 years | complications from type 1 diabetes mellitus | RRMS | Natalizumab | 6.0 | Ø | Ø | Ø | Ø |
| 8 | 69/F | 29 years | metastatic colon cancer | PPMS | None | 8.0 | Ø | Ø | Ø | Ø |
| 9 | 45/F | 16 years | pneumonia | PPMS | None | Unknown | Ø | Ø | Ø | Ø |
| 10 | 35/M | 12 years | MS | PPMS | Unknown | Unknown | Ø | Ø | Ø | Ø |
| 11 | 66/F | 32 years | MS | SPMS | None | 7.0 | Ø | Ø | Ø | Ø |
| 12 | 50/F | 13 years | MS | SPMS | Fingolimod | 7.0 | Ø | Ø | Ø | Ø |
| 13 | 95/F | 56 years | fulminant pulmonary edema | SPMS | None | 7.0 | Ø | Ø | Ø | Ø |
| 14 | 54/M | 26 years | pulmonary disease | SPMS | Copaxone | 7.0 | Ø | Ø | Ø | Ø |
Ø = no fibers myelinated with P0+ myelin; + = ≤5 fibers myelinated with P0+ myelin; ++ = 6–19 fibers myelinated with P0+ myelin; +++ = >20 fibers myelinated with P0+ myelin; MED = medulla; CSC = cervical spinal cord; TSC = thoracic spinal cord
Histology
LFB-PAS and solochrome cyanine staining for myelin were performed on 5um thick formalin-fixed paraffin-embedded (FFPE) sections to identify MS lesions and to aid in the classification of the lesion as remyelinating before commencing with immunohistochemistry (IHC). Remyelinating lesions were defined as areas with reduced density of pale myelin compared to the surrounding normal-appearing white matter10.
Immunohistochemistry
5um FFPE sections were deparaffinized with xylene, rehydrated in descending concentrations of ethanol to water, and heat-mediated antigen retrieval in 0.1M citric acid pH 6. Sections were blocked for endogenous avidin and biotin (Avidin/Biotin Blocking Kit, Vector Laboratories, SP-2001) and for endogenous peroxidases using 3% hydrogen peroxide to reduce non-specific staining. Staining with rabbit anti-P0 (produced by the laboratory of author Dr. Bruce Trapp at Cleveland Clinic Lerner Research Institute) was performed by incubation 1:250 overnight at 4°C. A biotinylated secondary antibody (Rockland, 611-706-125) was applied for 1 hour at room temperature, followed by streptavidin-HRP (Rockland, S000-03). HRP was developed using a DAB kit (BD Pharmingen, 550880). Sections were then counterstained with Shandon Instant Hematoxylin Kit (Thermo, 6765015), dehydrated, and cover-slipped with Permount mounting medium. To be considered P0+, lesions had to show at least one P0+ axon.
Immunofluorescence
5um thick FFPE sections were processed for immunohistochemistry. Rat anti-glial fibrillary acid protein (GFAP, Life Technologies 130300) diluted 1:100 in 5% horse serum, 0.1% Triton-X, and PBS was used to identify reactive astrocytes. Sections were also labeled with a P0 antibody to identify myelin produced by SchCs. Co-staining with rabbit anti-proteolipid protein (PLP, Abcam ab28486) diluted 1:100 was used to identify myelin produced by OLs. Co-staining with goat anti-Iba1 antibody (Novus, NB100–1028) diluted 1:250 was used to identify microglia. Secondary antibodies, with Alexa Fluor, conjugates 647 and 488, were used to target the primary host species. Two to five images of each region of interest were analyzed using MetaMorph 7.7 software to calculate the percentage of fluorescent intensity for the GFAP signal (pixels/mm2). The number of images analyzed was decided based on the size of the region of interest. For statistical analysis, the standard software package GraphPad Prism version 7.0 was used. 2-way ANOVA statistical test was performed with Bonferroni’s post hoc analysis.
Imaging
Histological and IHC stains were imaged using the Hamamatsu NanoZoomer (Alafi Neuroimaging Laboratory, Hope Center for Neurological Disorders, Washington University). Fluorescent images were acquired using a Nikon Eclipse 90ifluorescent and bright field microscope.
Results
Cerebral remyelination by Schwann cells in the index case
The index case was a 33-year-old male with an 11-year history of RRMS, who died from hypertrophic cardiomyopathy. MS disease onset was at age 22 years and characterized by bilateral leg numbness and ataxia. MRI performed at onset showed both brain and spinal cord lesions consistent with a diagnosis of MS. Cerebrospinal fluid analysis was positive for the intrathecal synthesis of IgG. He was started on interferon beta-1a treatment at diagnosis, and had no subsequent relapses or Gd-enhancing lesions. His last neurological exam (6 months before death) was reported as normal. At autopsy, macroscopic examination of the brain and the spinal cord showed a small gray gelatinous subpial lesion in the ventral pons, an area in the superior parietal lobe suspicious for a cortical MS plaque, and numerous sharp-bordered gelatinous gray plaques in a periventricular distribution in the frontal, parietal, temporal and occipital white matter. Routine histological stains were performed by the Washington University Department of Pathology and Immunology, including LFB-PAS. The neuropathologist (RES) noted LFB-PAS myelin staining that differed in color from CNS myelin in a cerebral periventricular white matter lesion, suggesting that remyelination may have been from SchCs (FIG. 1). P0 antibody was used to confirm the presence of PNS myelin in this lesion and to examine for the presence of SchC remyelination in the other 15 lesions identified and examined throughout the cerebrum, brainstem, and spinal cord of this patient. (FIG. 2). Co-staining for P0 and PLP showed that SchC remyelination was distinct from OL remyelination, and PLP+ myelin was more prevalent than P0+ myelin in remyelinating lesions (FIG. 3).
Figure 1. Demyelinating lesion in the index case.
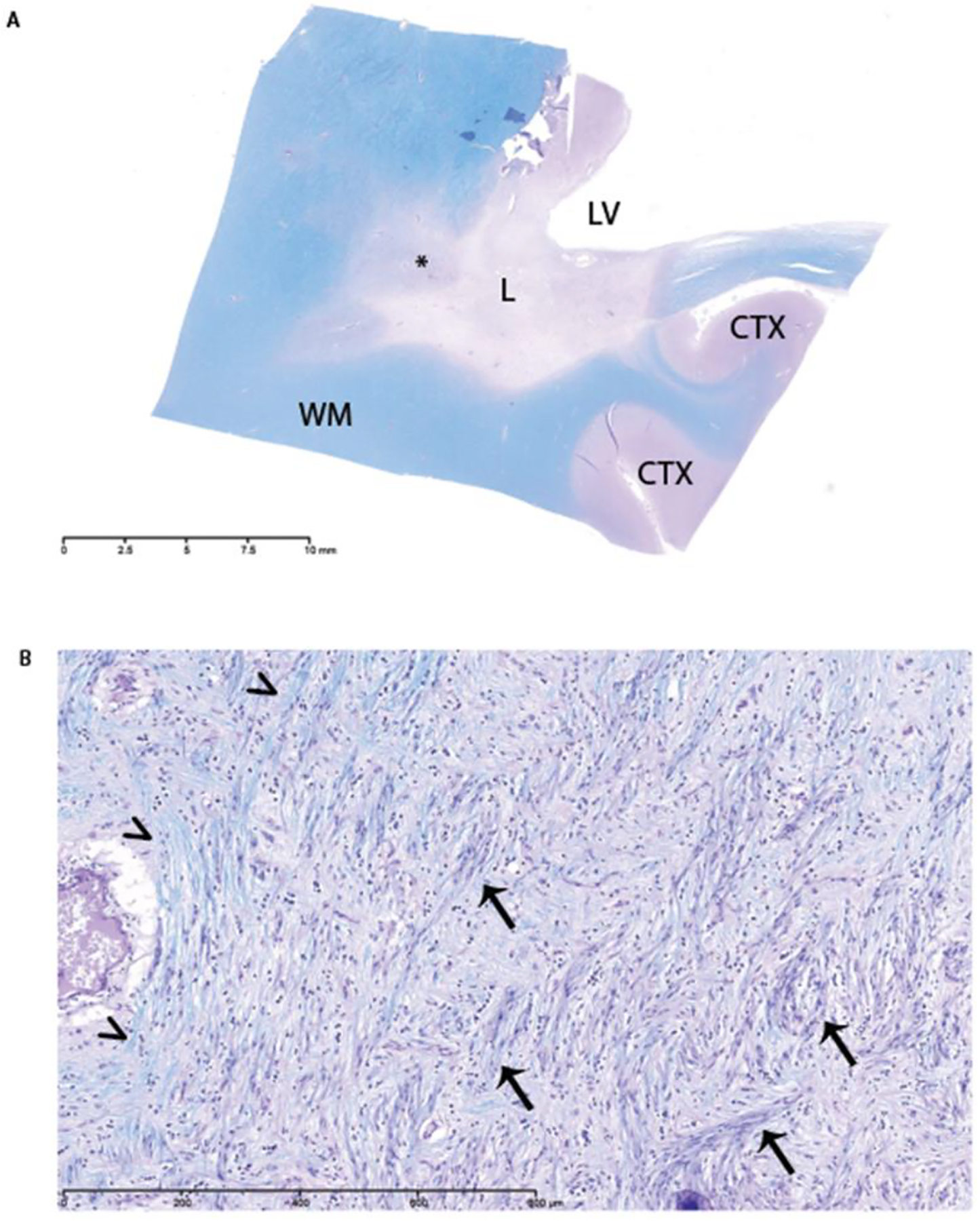
A) LFB-PAS staining of index case (Case 1) showing a large, periventricular white matter lesion in the cerebrum. LV=lateral ventricle, L=lesion, WM=normal appearing white matter, CTX=cortex, asterisk=region of interest for high magnification. B) High magnification of remyelinated lesion showing blue myelin (arrowheads) indicative of CNS myelin and purple myelin (arrows) indicative of PNS myelin.
Figure 2. Areas with P0+ staining in the cerebrum, pons, and spinal cord from index case (Index Case 1;
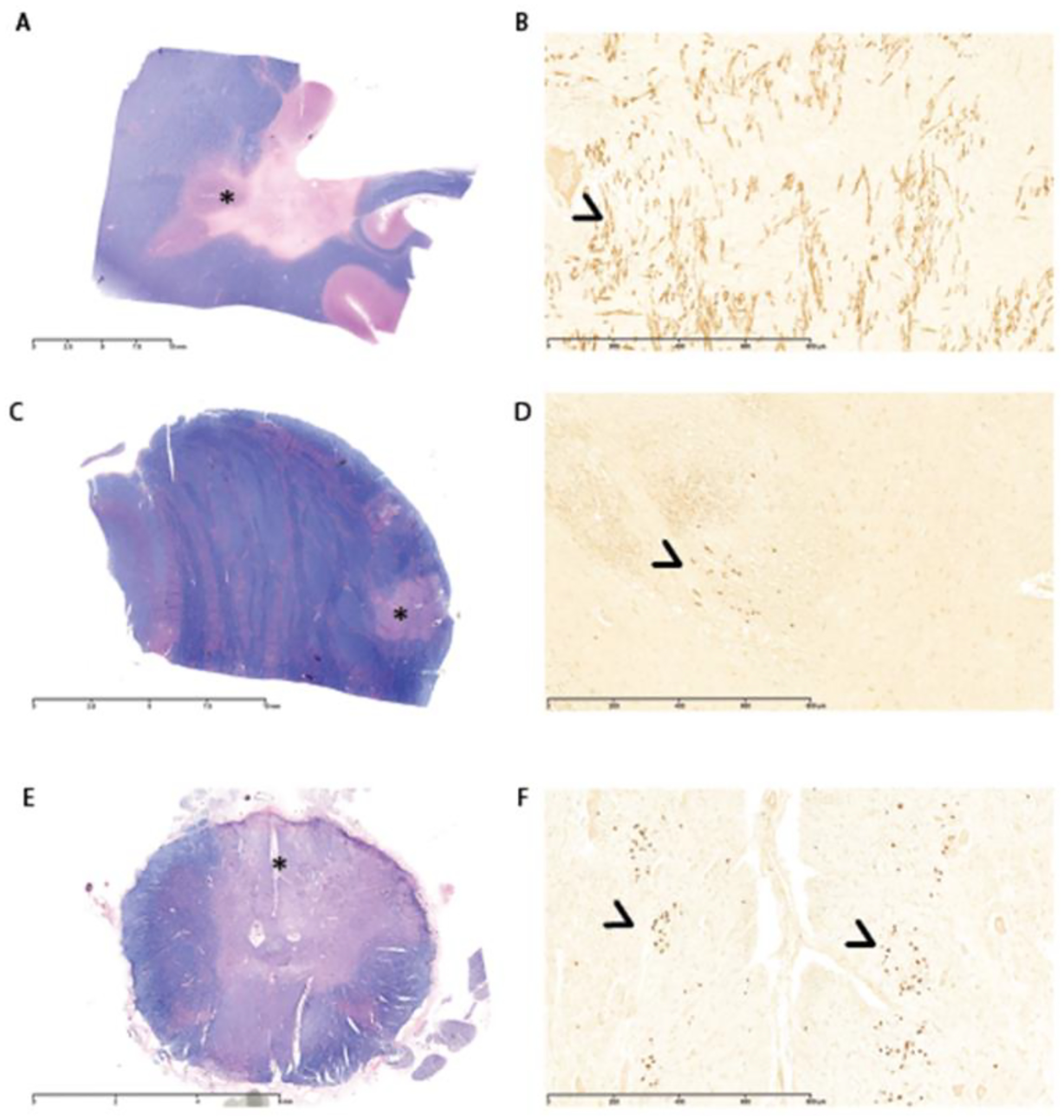
A, C, and E, respectively, show low magnification images). Tissues were stained with Solochrome Cyanine to evaluate areas of demyelination (lack of blue staining). Asterisks indicate regions of interest for high magnification images (B, D, and F), which show P0 staining in the cerebrum, pons, and spinal cord, respectively (arrowheads).
Figure 3. Concomitant expression of proteolipid protein (PLP) and P0 in remyelinating cerebral lesion (Index case 1).
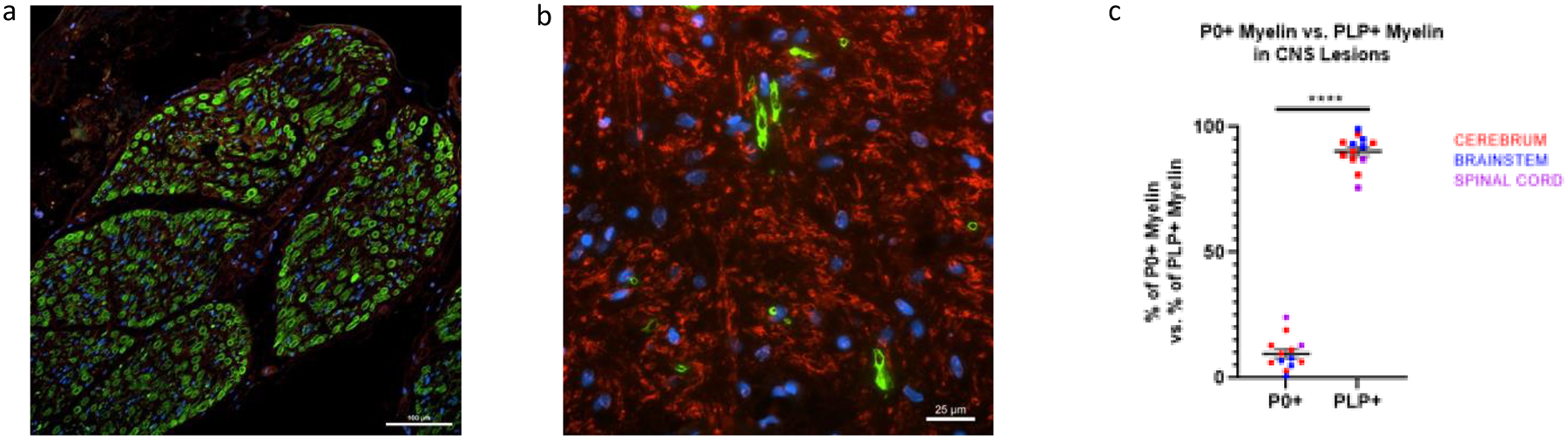
Peripheral nerve from Case 4 stained with antibody to P0 (green) and antibody to PLP (red), showing that antibody to P0 is specific to PNS myelin (20x magnification) (a). Remyelinating cerebral lesion from Case 1 showing the presence of anti-P0 (green) and anti-PLP (red) myelin (60x magnification) (b). The percentage of P0+ myelin vs PLP+ myelin in 6 lesions from 4 patients with P0+ myelin (thirteen 60x fields) was calculated as P0+ myelin/(P0+ myelin + PLP+ myelin) vs. PLP+ myelin/(P0+ myelin + PLP+ myelin). P0+ myelin (mean percentage = 9.74%) was significantly less present than PLP (mean=90.26%) in remyelinating lesions of the CNS.
Schwann Cell remyelination in post-mortem brain and spinal cord MS tissues
To examine whether SchC remyelination of the brain and other CNS regions was widespread among remyelinated MS lesions in other cases, we examined additional MS tissues maintained in the Neuroimmunology Section Tissue Repository at Washington University. Sixty-four (64) MS lesions from 14 autopsies, including the index patient, were examined. 35.9% of these lesions were positive for P0 staining (23 P0+ lesions/64 lesions). 42% of the analyzed spinal cord lesions had P0+ SchC remyelination in their spinal cords (6/18 autopsies), 21% of analyzed brainstem lesions had P0+ SchC remyelination in the brainstem (3/14 autopsies), and 21% of analyzed cerebral lesions had P0+ SchC remyelination in the cerebrum (3/14 autopsies) (FIG. 4). In conclusion, we observed SchC remyelination in 6 out of 14 cases analyzed. Eight cases showed no P0+ staining in either the brain or the spinal cord. SchC remyelination was most common in the spinal cord (< 20% of total myelin in the lesion) but was also noted, as scattered foci (< 10% of total myelin in the lesion), in the brainstem and cerebrum of 4 of the analyzed cases. Based on the available information, there were not statistical significant differences in age and gender between the P0+ and the P0− cases. It was noted that the 6 autopsies showing evidence of remyelination by SchC had an average disease duration of 13.5 years (range 6–22 years) before death, whereas the average duration of disease was 24.9 years (range 12–56 years) for those without evidence of SchC remyelination.
Figure 4: Percentage of MS cases analyzed that had any P0+ cells in their cerebrum, brainstem, and spinal cord lesions.
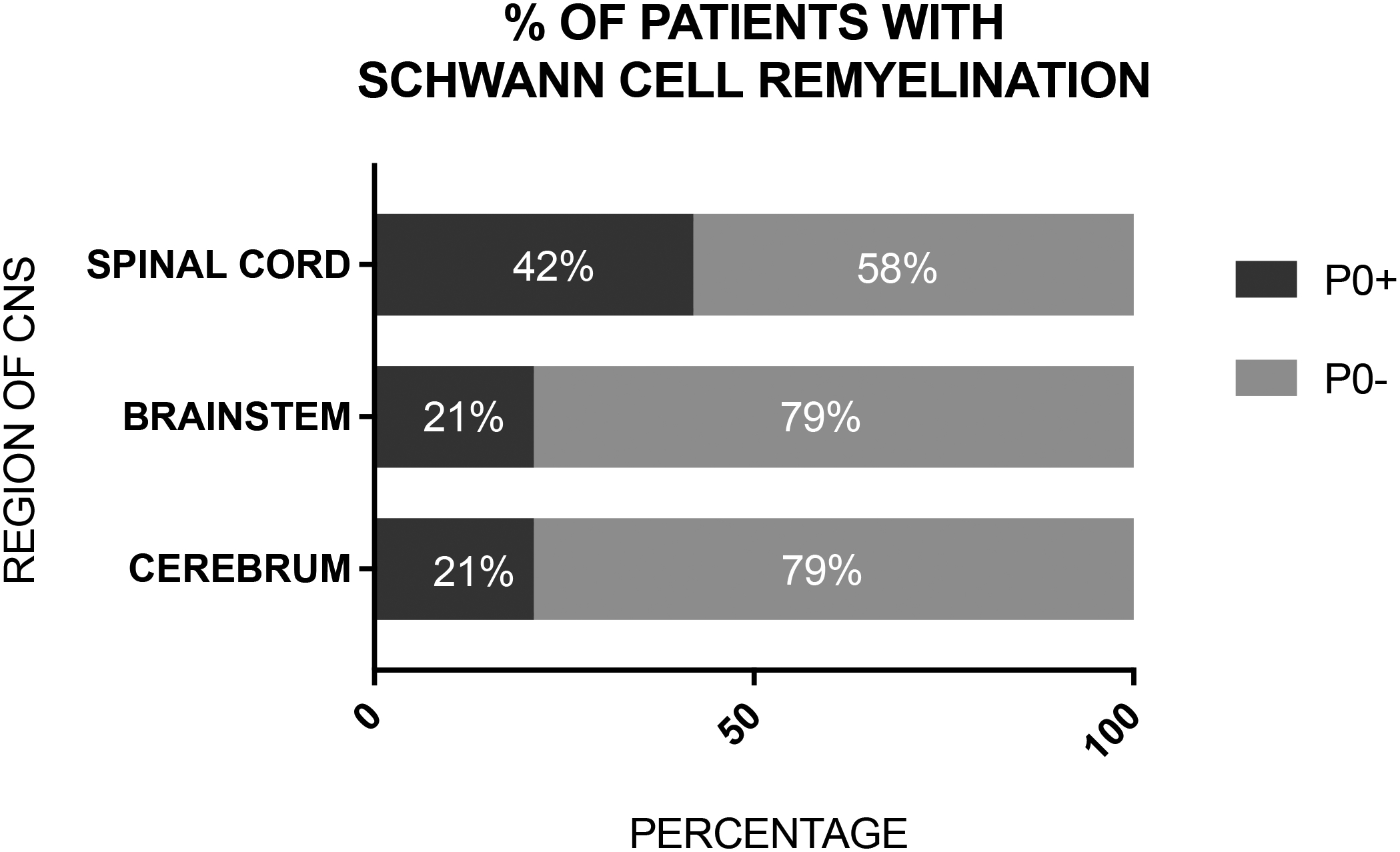
Forty-two percent of the cases (6/14 total cases) had P0+ cell remyelination in the spinal cord, while 21% of the cases (3/14) had P0+ remyelination in the brainstem and cerebrum.
Schwann cell remyelination occurs frequently in proximity to blood vessels
While analyzing the P0+ lesions, we observed P0+ fibers in proximity to blood vessels. We analyzed the 23 P0+ lesions and found that 73.9% (17/23 lesions) had P0+ fibers that were perivenular, while 21.6% of lesions with SchC remyelination did not have perivascular P0+ fibers (6/23 total lesions) (FIG. 5). Perivascular was defined as adjacent to the vessel wall and within a 100um radius around the lumen of the vessels, which were all venules.
Figure 5: Solochrome Cyanine (A) and P0 antibody staining (B) of a remyelinated spinal cord lesion from Case 5 showing perivascular P0+ Schwann cell remyelination.
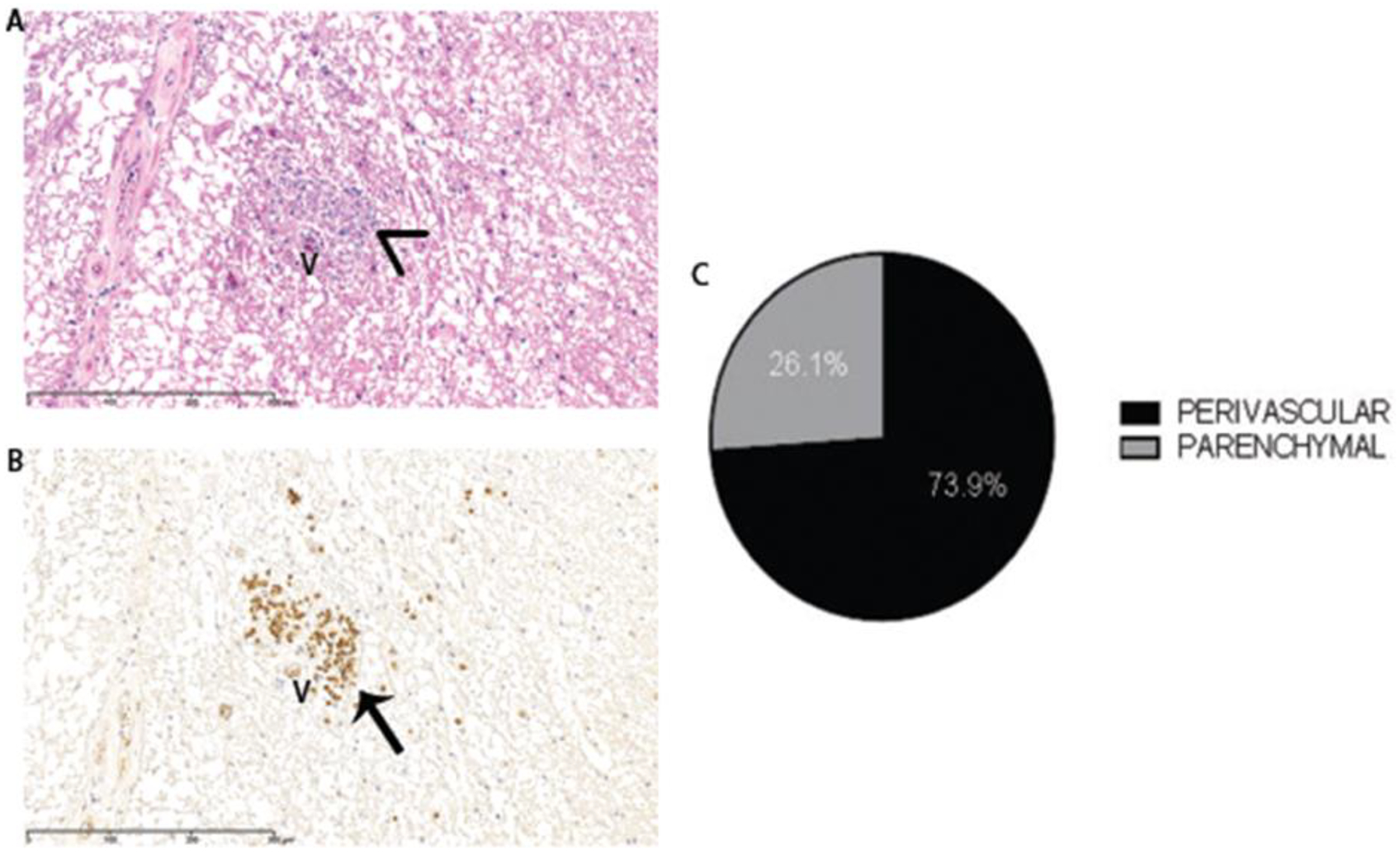
V=venule. Arrowhead shows solochrome cyanine-stained myelin wrapped around axons, and the arrow shows the same myelin positive for the P0 antibody. 73.9% of the P0+ lesions analyzed (17/23 total lesions) had perivascular SchC remyelination (C).
P0+ remyelinated MS lesions have fewer GFAP+ reactive astrocytes compared to P0-negative lesions
We examined for the presence of reactive astrocytes, defined by GFAP reactivity, in the cerebrum, brainstem, and spinal cord of autopsied cases. Two to five images from 3 lesions with P0+ remyelination and 5 lesions without P0 staining from each CNS region were examined. Lesions with P0+ remyelination had lower GFAP fluorescence intensity when compared to lesions without SchC remyelination (FIG. 6). This differential finding was statistically significant in the spinal cord and brainstem lesions (P<0.05 and P<0.01, respectively, 2-way ANOVA with Bonferroni’s post hoc correction), while in the cerebrum, the difference was not statistically significant (P=0.33). Iba1 was used to identify microglia. However, no differences in the number or morphology of microglia were noted in lesions containing P0+ myelin versus those without.
Figure 6: Representative images from Patient 2 and Patient 11 (A and B, respectively) of GFAP+ reactive astrocytes (red), P0+ myelin (green), and DAPI (blue) in the anterior funiculus of spinal cord lesions.
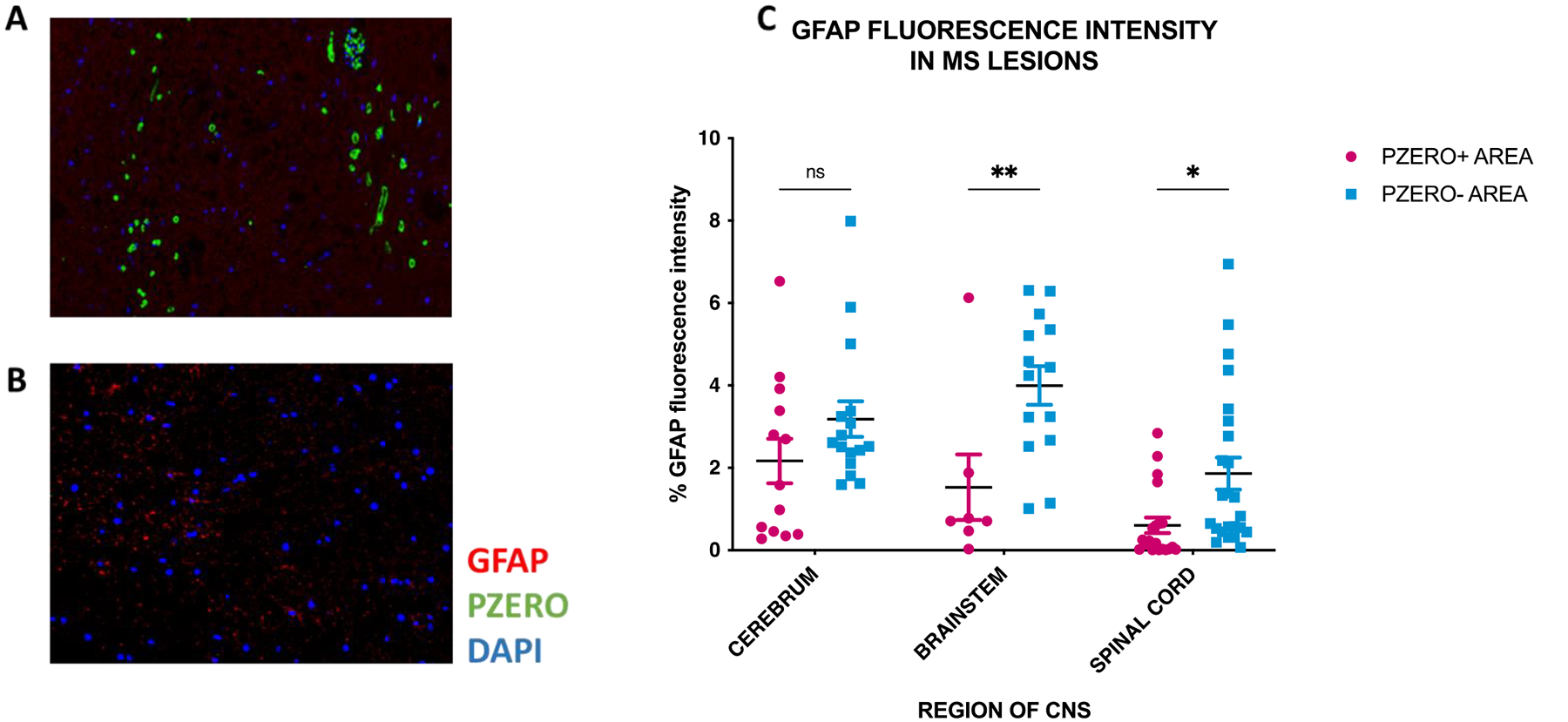
The lesion in A shows substantial SchC remyelination and lack of GFAP+ astrocytes. On the contrary, lesion in B does not show SchC remyelination (no evidence of P0+ myelin), but numerous GFAP+ reactive astrocytes are evident. Nine lesions with P0+ myelin (3 from each CNS region) and 15 lesions negative for P0+ myelin (5 from each CNS region) were analyzed for GFAP+ fluorescence intensity, showing a significant decrease in reactive astrocytes in lesions with SchC remyelination in the spinal cord and brainstem (P<0.01 and P<0.05, respectively, 2-way ANOVA with Bonferroni’s post hoc correction) (C).
Discussion
Demyelination inhibits axonal conduction and if remyelination fails, demyelination can ultimately lead to axonal and neuronal cell body degeneration11. Demyelinated axons have increased energy demands and lack the metabolic support provided by myelin12. Unmet energy demands can increase axonal Ca++ to toxic levels, resulting in axon degeneration. This is thought to cause permanent disability accumulation in pwMS13. Remyelination of MS lesions is highly variable, extensive in some cases, and virtually absent in others3,4,12. Failure to remyelinate MS lesions can be ascribed to impaired recruitment of oligodendrocyte precursor cells (OPCs) into a lesion or an inability to differentiate and mature into remyelinating oligodendrocytes (OLs)12,14. The absence of axonal activity, the presence of axonal inhibitors such as LINGO1 and sulphated proteoglycans such as hyaluronan and versican, and an excessive pro-inflammatory response have all been hypothesized to create a microenvironment unfavorable to OL-mediated remyelination15–22. SchC remyelination has been suggested to occur preferentially where OLs are absent or have been killed by the demyelinating insult23. Two hypotheses have been put forth regarding how SchCs are located in the CNS and remyelinate axons. One hypothesis is that SchCs migrate from the PNS to the CNS to aid in remyelination24. The other hypothesis is that OPCs found throughout the CNS can differentiate into SchCs25. While OPCs are the cells that differentiate into OL during remyelination26, they are also known to acquire expression of P0, and aid in the remyelination of demyelinated CNS lesions in animal models25,27. The relevance of SchC remyelination in MS is controversial28. Importantly, it is unknown if remyelination of axons by SchCs is as effective as remyelination of axons by OLs in restoring the ability to transmit neuronal signals in the CNS. It is possible, however, that SchC myelin is resistant to a second wave of demyelination in the MS spinal cord as there is evidence that the inflammatory response in demyelinating lesions did not damage P0+ myelin sheaths when SchC remyelination occurred5.
Remyelination by SchCs has been reported in the spinal cords of MS patients. It has been hypothesized to occur due to the proximity of the spinal cord region to SchCs in the dorsal and ventral nerve roots. To our knowledge, remyelination of the cerebrum by SchCs has not been previously reported in humans. Here we have analyzed the occurrence of P0+ myelin in 64 lesions from 14 MS autopsies, including spinal cord lesions, brainstem, and cerebral lesions. Consistent with previous reports, we observed remyelination by SchCs in the spinal cords of 6 of 14 individual autopsies. We also report remyelination by SchCs, characterized by P0+ myelin, in the brainstem and cerebral regions in all 6 autopsies with SchC remyelination in the spinal cord (Table 1). Nearly three-quarters of the lesions with SchC remyelination displayed P0+ fibers in a perivenular location. This localization was of special interest due to prior reports that SchCs can emerge in the CNS from perivascular autonomic nerves during disease-induced demyelination29.
We also observed SchC remyelination to occur in areas depleted of GFAP+ astrocytes. This is in accord with animal model studies of chemical-induced demyelination in which SchC remyelination was observed after SchC transplantation into astrocyte-depleted lesions30. In that study, adult human sural nerve SchCs were transplanted into the X-irradiation/ethidium bromide lesioned dorsal columns of immunosuppressed Wistar rats. After 3 to 5 weeks, a typical SchC remyelination pattern was observed in previously astrocyte-depleted demyelinated areas30.
Currently, available therapies can control MS inflammation manifesting as relapses and contrast-enhancing MS lesions but have modest or no effects on progressive pathobiology. New pro-remyelinating strategies to promote tissue repair and counteract long-term axonal damage are greatly needed12.The potential of SchCs to contribute to CNS repair has been reported in several cases of traumatic injury31 as well as in leukodystrophy32, and in spinal cord of MS5.
Using genetically engineered mice, Zawadzka et al.25, reported that CNS OPCs can differentiate into SchCs and contribute to remyelination under specific environmental cues. It is still unclear whether the molecular mechanisms are involved in regulating the OPCs CNS-to-PNS fate-switching. Several lines of evidence indicate that SchCs are mostly found in regions lacking reactive astrocytes30,33. Astrocytes are known to play a crucial role in regulating which type of remyelination occurs via STAT3 expression34. Thus, astrocyte-depleted areas may be more permissive to SchC differentiation of CNS OPCs. Moreover, using a chemical-induced mouse model of demyelination, it has been reported that perivascular niches are involved in regulating bone morphogenetic protein (BMP) and wingless/integrated (WNT) signaling to support alternative differentiation of OPCs into SchCs33. It was also shown that reactive astrocytes inhibit the BMP/Wnt pathway that drives the differentiation of SchC from OPCs (Ulanska-Poutanen et al., 2018). This is in line with our post-mortem analyses in which SchCs were predominantly found in perivenular areas, in close contact with the blood vessels, and areas lacking reactive astrocytes expressing GFAP. A recent population of perivascular OPCs (pvOPCs) has been described to be involved in pro-angiogenic processes35 and the maintenance of blood-brain-barrier integrity36. Therefore, it might be hypothesized that these pvOPCs under specific molecular cues, perhaps arising from the vasculature in the absence of astrocytes, may undergo a fate-switching to differentiate into SchCs with the added implication that pvOPCs might be a new target for drug development for CNS remyelination. This would be especially favorable in the setting of MS, where PNS myelin is not affected37
Fundings:
BDT was supported by the NIH grant R35NS09730; LG was supported by the NMSS post-doctoral fellowship FG-1907-34474.
Competing Interests statement:
LP receives support from Alector. AHC has received fees for consulting or participating in advisory boards for: Biogen, Bristol-Myers Squibb, EMD Serono (affiliate of Merck KgaA), Genentech (Roche), Horizon Pharmaceuticals, Janssen (J&J), Jazz Pharmaceuticals, Novartis, Octave, and TG Therapeutics and AHC serves on scientific advisory boards for EMD Serono, Novartis and Genentech. AHC was supported by The Manny & Rosalyn Rosenthal - Dr. John L. Trotter MS Center Chair in Neuroimmunology of The Foundation for Barnes-Jewish Hospital. BDT was supported by the NIH grant R35NS09730; LG was supported by the NMSS post-doctoral fellowship FG-1907-34474.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement:
data sharing not applicable to this article as no datasets were generated or analyzed during the current study
REFERENCES
- 1.Franklin RJM, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–855. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki M, Black JA, Lankford KL, Tokuno HA, Waxman SG, Kocsis JD. Molecular Reconstruction of Nodes of Ranvier after Remyelination by Transplanted Olfactory Ensheathing Cells in the Demyelinated Spinal Cord. Journal of Neuroscience. 2006;26(6):1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramow S, Frischer JM, Lassmann H, et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133(10):2983–2998. [DOI] [PubMed] [Google Scholar]
- 4.Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33(3):277–287. [DOI] [PubMed] [Google Scholar]
- 5.Itoyama Y, Webster HD, Richardson EP, Trapp BD. Schwann cell remyelination of demyelinated axons in spinal cord multiple sclerosis lesions. Ann Neurol. 1983;14(3):339–346. [DOI] [PubMed] [Google Scholar]
- 6.Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P. Smoking as a risk factor for multiple sclerosis. Multiple Sclerosis Journal. 2013;19(8):1022–1027. [DOI] [PubMed] [Google Scholar]
- 7.Feigin I, Cravioto H. A Histochemical Study of Myelin. J Neuropathol Exp Neurol. 1961;20(2):245–254. [DOI] [PubMed] [Google Scholar]
- 8.Fraher JP. The CNS-PNS transitional zone of the rat. Morphometric studies at cranial and spinal levels. Prog Neurobiol. 1992;38(3):261–316. [DOI] [PubMed] [Google Scholar]
- 9.Beal JA, Hall JL. A light microscopic study of the effects of X-irradiation on the spinal cord of neonatal rats. J Neuropathol Exp Neurol. 1974;33(1):128–143. [DOI] [PubMed] [Google Scholar]
- 10.Patrikios P, Stadelmann C, Kutzelnigg A, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129(Pt 12):3165–3172. [DOI] [PubMed] [Google Scholar]
- 11.Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131(Pt 6):1464–1477. [DOI] [PubMed] [Google Scholar]
- 12.Plemel JR, Liu WQ, Yong VW. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov. 2017;16(9):617–634. [DOI] [PubMed] [Google Scholar]
- 13.Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci. 1992;12(2):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruchot J, Weyers V, Göttle P, et al. The Molecular Basis for Remyelination Failure in Multiple Sclerosis. Cells. 2019;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jepson S, Vought B, Gross CH, et al. LINGO-1, a transmembrane signaling protein, inhibits oligodendrocyte differentiation and myelination through intercellular self-interactions. J Biol Chem. 2012;287(26):22184–22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautier HOB, Evans KA, Volbracht K, et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat Commun. 2015;6:8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawji KS, Yong VW. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin Dev Immunol. 2013;2013:948976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang A, Staugaitis SM, Dutta R, et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;72(6):918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghorbani S, Yong VW. The extracellular matrix as modifier of neuroinflammation and remyelination in multiple sclerosis. Brain. 2021;144(7):1958–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pu A, Stephenson EL, Yong VW. The extracellular matrix: Focus on oligodendrocyte biology and targeting CSPGs for remyelination therapies. Glia. 2018;66(9):1809–1825. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Zhang CJ, Martin BN, et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat Commun. 2017;8:15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghorbani S, Jelinek E, Jain R, et al. Versican promotes T helper 17 cytotoxic inflammation and impedes oligodendrocyte precursor cell remyelination. Nat Commun. 2022;13(1):2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felts PA, Woolston AM, Fernando HB, et al. Inflammation and primary demyelination induced by the intraspinal injection of lipopolysaccharide. Brain. 2005;128(Pt 7):1649–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franklin RJ, Blakemore WF. Requirements for Schwann cell migration within CNS environments: a viewpoint. Int J Dev Neurosci. 1993;11(5):641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zawadzka M, Rivers LE, Fancy SPJ, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6(6):578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fancy SPJ, Zhao C, Franklin RJM. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 2004;27(3):247–254. [DOI] [PubMed] [Google Scholar]
- 27.Chen CZ, Neumann B, Förster S, Franklin RJM. Schwann cell remyelination of the central nervous system: why does it happen and what are the benefits? Open Biol. 2021;11(1):200352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cayre M, Falque M, Mercier O, Magalon K, Durbec P. Myelin Repair: From Animal Models to Humans. Front Cell Neurosci. 2021;15:604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kegler K, Spitzbarth I, Imbschweiler I, Wewetzer K, Baumgärtner W, Seehusen F. Contribution of Schwann Cells to Remyelination in a Naturally Occurring Canine Model of CNS Neuroinflammation. PLoS One. 2015;10(7):e0133916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shields SA, Blakemore WF, Franklin RJ. Schwann cell remyelination is restricted to astrocyte-deficient areas after transplantation into a demyelinated adult rat brain. J Neurosci Res. 2000;60(5):571–578. [DOI] [PubMed] [Google Scholar]
- 31.Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 32.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192(2):384–393. [DOI] [PubMed] [Google Scholar]
- 33.Ulanska-Poutanen J, Mieczkowski J, Zhao C, et al. Injury-induced perivascular niche supports alternative differentiation of adult rodent CNS progenitor cells. Chao MV, Bronner ME, eds. Elife. 2018;7:e30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monteiro de Castro G, Deja NA, Ma D, Zhao C, Franklin RJM. Astrocyte Activation via Stat3 Signaling Determines the Balance of Oligodendrocyte versus Schwann Cell Remyelination. Am J Pathol. 2015;185(9):2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kishida N, Maki T, Takagi Y, et al. Role of Perivascular Oligodendrocyte Precursor Cells in Angiogenesis After Brain Ischemia. J Am Heart Assoc. 2019;8(9):e011824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanti A, Nagy C, Maitra M, Wakid M, Turecki G, Mechawar N. A Post-Mortem Investigation of Perivascular Oligodendrocyte Precursor Cells in the Prefrontal Cortex of Major Depressed Patients. Biol Psychiatry. 2020;87(9, Supplement):S91. [Google Scholar]
- 37.Kocsis JD, Waxman SG. Schwann cells and their precursors for repair of central nervous system myelin. Brain. 2007;130(Pt 8):1978–1980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
data sharing not applicable to this article as no datasets were generated or analyzed during the current study


