Abstract
Introduction:
Phantoms and simulators are widely accepted methods to gain valuable experience and confidence for inexperienced trainees prior to seeing their patient and for refining their skills. A phantom model that is durable, simple, and inexpensive to produce and use would be ideal to train practitioners in ultrasound guided fine needle aspiration biopsy (USFNA) technique.
Methods and materials:
In this study, we systematically compared several low-cost phantom models including gelatin, extra firm tofu, canned cooked pork, ballistics gel and chicken breast for their haptic properties, echogenicity, teaching utility and overall performance based on a Likert scale (1-5; 5=best). Nine cytopathologists and cytopathology fellows who perform FNA regularly evaluated these models and completed the survey.
Results:
The gelatin phantom with a gelatin to water ratio of 1:8 by weight was found to be the best for USFNA practice and overall performance, followed by the 1:10 gelatin phantom. Tofu and chicken breast phantoms were also good low-cost alternatives that needed only a few minutes of total preparation time.
Conclusions:
Low-cost, homemade phantoms can serve as excellent alternatives to commercial phantoms for practicing and teaching USFNA.
Keywords: Fine needle aspiration biopsy, ultrasound, phantom, training
Introduction
Ultrasound guided fine needle aspiration biopsy (USFNA) is a minimally invasive tissue sampling method that has few procedure-related complications. However, the accuracy of FNA is dependent on the practitioner’s experience and skill1. Therefore, hands-on training with feedback is critical in gaining competence and confidence in USFNA technique2. US phantoms are an excellent and widely accepted method of introducing USFNA to inexperienced trainees prior to seeing their patient and for refining their skills 3–6. Although commercial phantoms are available, they are expensive and often retain needle track marks. Homemade phantoms that are simple and inexpensive to produce are ideal for training practitioners in USFNA technique. The literature has described phantoms made from a wide range of materials, including gelatin, canned cooked pork, chicken, tofu, silicone, and ballistics gel . However, the recipes for homemade phantoms vary widely, even when using similar materials, and many are optimized for vascular access training. Therefore, in this study, we aimed to compare several simple and low-cost phantoms for USFNA training, focusing on haptic properties, echogenic qualities, usefulness for practice, cost, and the time to create each phantom.
Materials and Methods
We compared homemade phantom models made of ballistics gel, gelatin, chicken breast, extra firm tofu, canned cooked pork as well as a commercial phantom (Blue Phantom™ Soft Tissue Biopsy Ultrasound Medical Training Block Model, CAE HealthCare, Sarasota, FL). All homemade phantom models used materials readily available at the supermarket except for the model made of ballistics gel. Although there are no strict specifications regarding target placement, we used the following general guidelines: Each phantom contained at least three targets at various depths, ranging from approximately 0.5cm from the superficial surface to immediately adjacent to the deep surface, at least 2.5 cm from the peripheral edges of the phantom, and at least 0.5 cm from other targets (Figures 1A and B).
Figure 1:
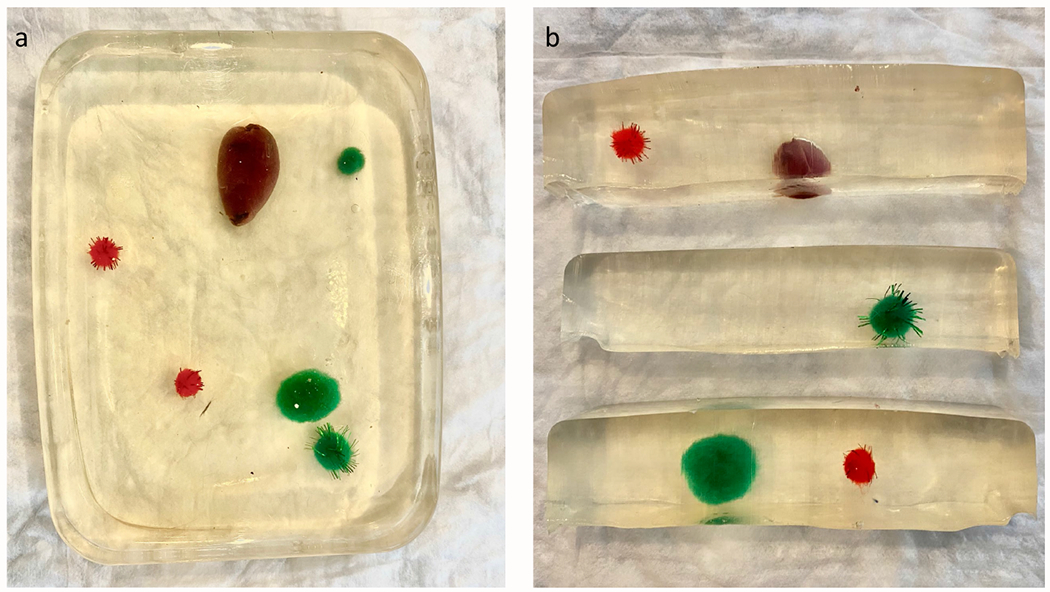
Images of gelatin phantom model from above (A) and cross section (B) to illustrate the placement of targets.
Ballistics gel phantom
We cut up 16 oz (454 g) of Gelatin #0, synthetic gelatin/ballistics gel (Clear Ballistics/Humimic Medical, Greenville, SC) into 1-inch cubes and placed it into oven-safe glassware, which also served as the phantom mold. The oven was preheated to 260 °F (127 °C) and the glassware was heated until the ballistics gel was completely melted (approximately 1 hour)9. Craft pom poms of variable sizes were inserted as targets, and the ballistics gel was heated for another 30 minutes until the surface was smooth. It was noted that any significant manipulation introduced unwanted air bubbles, which are known to reflect ultrasound waves. The phantom was then allowed to cool at room temperature for approximately 2 hours and removed from the mold.
Gelatin phantoms
We used Knox unflavored powdered gelatin (Kraft Heinz Foods Company, Chicago, IL) readily available at the supermarket and created serial mixtures, with gelatin to water ratios of 1:1, 1:2, 1:3, 1:4, 1:8, 1:10, 1:12, and 1:16 by weight in grams, in 50ml conical tubes. In each conical tube, 25ml of boiling water was added to the powdered gelatin and mixed until the solution was completely dissolved. The conical tubes were then chilled for at least 2 hours. Based on haptic properties (texture and firmness), two of the authors (XZ and DLN) then selected the 1:8 and 1:10 gelatin to water ratio phantoms to compare with the remaining phantom model types. For the full-sized phantom models, 500 ml of boiling water was added to 62.5g of gelatin for the 1:8 gelatin model, and to 50g of gelatin for the 1:10 gelatin model in a standard plastic good storage container. Approximately five drops of food coloring were added. The solution was mixed until completely dissolved. Various targets, including pitted olives and craft pom poms were inserted into the mold. The phantoms were then chilled for 4 hours until solid. Because the targets can be difficult to place when the gelatin is still liquid, the phantom can be chilled to a semi-solid state and then targets can be placed. If one is not satisfied with the placement of the targets after the phantom has solidified, the gelatin can also be re-heated at 10 second intervals until semi-solid so that the targets can be repositioned.
Chicken breast, extra firm tofu, and canned cooked pork phantoms
A knife was used to create a slit in each phantom and various targets were inserted. Cooked black beans and olives were used as targets for the chicken breast. Only cooked black beans were used as targets for the extra firm tofu and canned cooked pork (Hormel Foods, LLC, Austin, MN), since larger targets would have disrupted the integrity of the phantom.
All phantoms were wrapped in two layers of plastic cling wrap. Total cost and time to create each phantom was documented.
Nine cytopathologists and cytopathology fellows who regularly perform USFNA evaluated the homemade phantoms as well as the commercial phantom by identifying targets using the ultrasound (GE Logiq E9, Boston, MA) and then performing an ultrasound guided fine needle aspiration biopsy on a target using a 1 inch 25-gauge needle attached to a 10 ml syringe. Participants were allowed to adjust the ultrasound machine settings to optimize visualization of the needle and targets. Each participant filled out a survey based on their USFNA experience on the test phantoms in comparison to real-life USFNA experience on patients which served as the gold standard. The survey included the following parameters: overall haptic property, echogenic quality, usefulness in teaching and practicing USFNA, and overall impression based on a Likert scale (1-5; 5=best). Medians were determined for each graded quality of the phantom. Haptic properties were evaluated based on the similarity to real human tissue when touching the phantoms—primarily elasticity and firmness--as well as the resistance felt when inserting the needle in the phantoms. Echogenic quality was evaluated based on the ability to visualize the targets as well as needle clearly on ultrasound imaging without obscuring echogenic artifacts.
This project was reviewed by the Memorial Sloan Kettering Cancer Center Institution Review Board and determined to be a quality improvement project and did not constitute as human subjects research.
Results
Overall chicken breast, extra firm tofu and canned cooked pork phantoms took the least amount of active time and effort, and overall, can be made ad hoc for USFNA training (Table 1). All three phantoms could be made without special equipment and would cost less than $10 to create, but all were perishable and could only be used for a few days. The time to create the ballistics gel phantom and gelatin phantoms were similar but had different special handling requirements and would need to be made ahead of time for USFNA training. The ballistics gel phantom required an oven, and any manipulation would introduce air bubbles which was only minimally reduced by heating. The gelatin phantoms required special equipment to boil water and a refrigerator to chill the phantoms until firm. Out of all the phantoms, the ballistics gel phantom was the most durable and only modestly more expensive than the other food-based phantoms.
Table 1:
Median scores, time, cost, durability and rank of each phantom
| Commercial phantom | Ballistics gel | Gelatin 1:8 | Gelatin 1:10 | Chicken breast | Extra firm tofu | Canned cooked pork | |
|---|---|---|---|---|---|---|---|
| Haptic quality median (range, IQR) | 3 (2-4, 1) | 3 (2-4, 1) | 4 (3-5, 1) | 4, (3-5, 1) | 4, (2-5, 1) | 3, (2-5, 1) | 2 (1-4, 1) |
| Echogenic quality median (range, IQR) | 4 (3-5, 1) | 3 (1-5, 2) | 5 (4-5, 1) | 5 (4-5, 1) | 4 (2-5, 0) | 4 (2-4, 1) | 1 (1-4, 0) |
| Teaching utility median (range, IQR) | 4 (4-5, 1) | 4 (2-4, 1) | 5 (4-5, 1) | 4 (4-5, 1) | 3 (2-5, 1) | 3 (2-5, 0) | 1 (1-3, 0) |
| Overall impression median (range, IQR) | 4 (4-5, 0) | 4 (2-4, 2) | 5 (4-5, 1) | 4 (4-5, 0) | 3 (2-4, 1) | 3 (2-4, 0) | 1 (1-3, 1) |
| Active time | N/A | 20 min | 15 min | 15 min | <5 min | <5 min | <5 min |
| Total time | N/A | 3.5 hours | 4 hours | 4 hours | <5 min | <5 min | <5 min |
| Approximate total cost | $595 | $30 | $8 | $6.40 | $3.50 | $2.25 | $3.80 |
| Materials cost (oz) | N/A | $1.87/oz | $3.59/oz | $3.59/oz | $0.21/oz | $.15/oz | $0.32/oz |
| Shelf-life | No limit* | No limit* | 1 week | 1 week | 1-2 days | 1-2 days | 1-2 days |
| Overall rank | 2 | 4 (tie) | 1 | 3 | 6 | 4 (tie) | 7 |
These phantoms have an extended and indeterminate shelf life of at least several years.
For overall haptic quality, the 1:8 gelatin phantom, 1:10 gelatin phantom, and chicken breast all had a median score of 4 (Table 1, Figure 2A). The canned cooked pork had the worst haptic quality ratings, with a median of 2. The commercial phantom and ballistics gel had modest median scores of 3 because it was felt that they provided too much resistance compared to human tissue. The 1:8 and 1:10 gelatin phantom models both had median scores of 5 for echogenic quality (Table 1, Figures 2B, 3A–F), while chicken breast, extra firm tofu and commercial phantom had median scores of 4. Canned cook pork again had the worst ratings for echogenic quality with a median score of 1; in which the targets could not be visualized at all, even when superficial. As a comparison, we included images of the current state of our commercial ultrasound phantom, which has numerous track marks obscuring the targets (Figures 4A and B).
Figure 2:
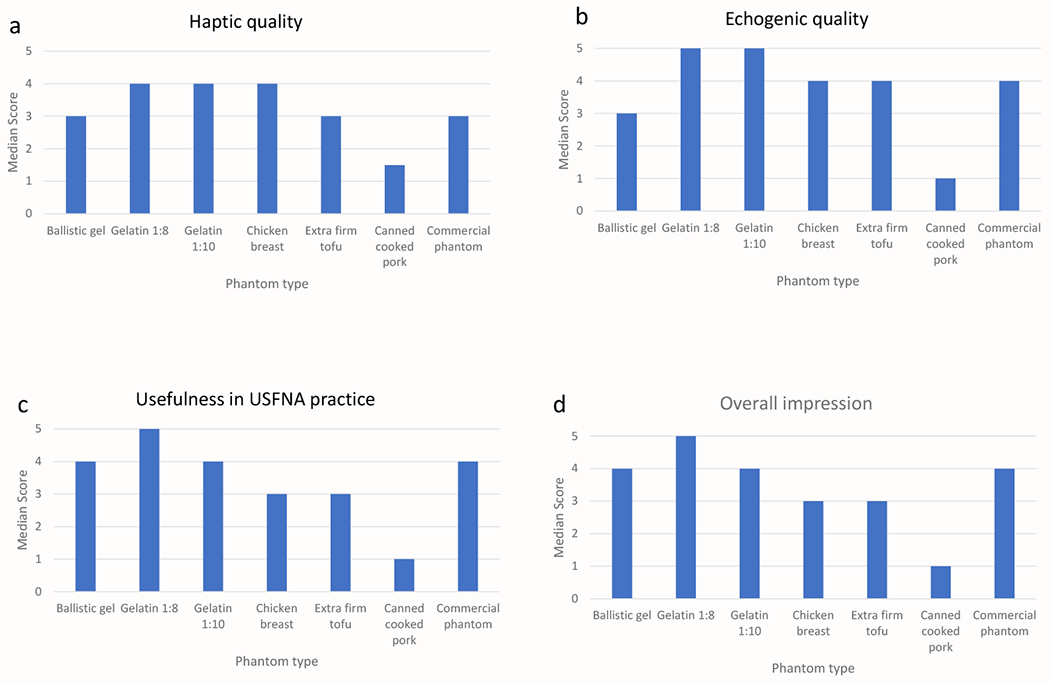
Median scores of each homemade phantom models for haptic quality (A), echogenic quality (B), usefulness in USFNA practice (C), as well as overall impression (d) in USFNA training.
Figure 3:
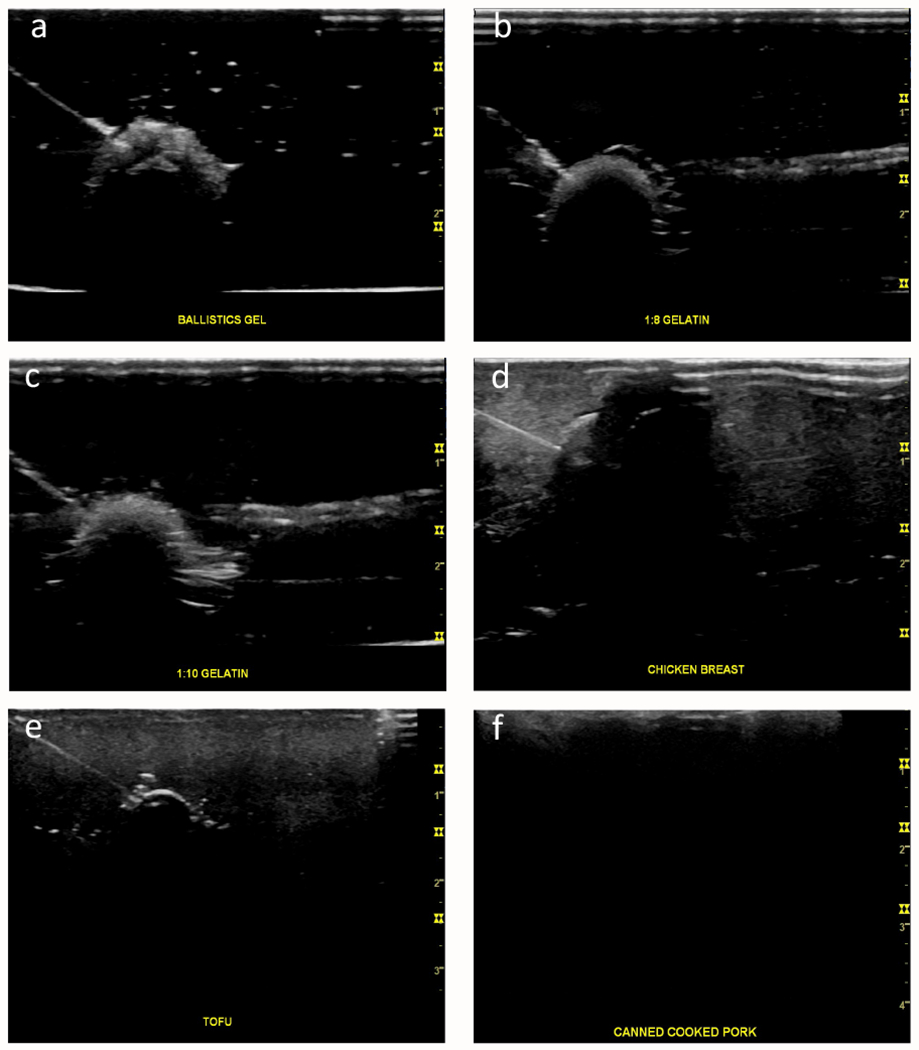
Illustration of the echogenic quality of phantom models for visualizing various targets and needle path in each homemade phantoms on ultrasound images. A) Ballistics gel with a craft pom pom. B) Phantom made 1:8 gelatin to water ratio with a pitted olive. C) phantom made 1:10 gelatin to water ratio with a pitted olive. D) Chicken breast with a pitted olive. E) extra firm tofu with a cooked black bean. F) Canned cooked pork with a cooked black bean.
Figure 4:
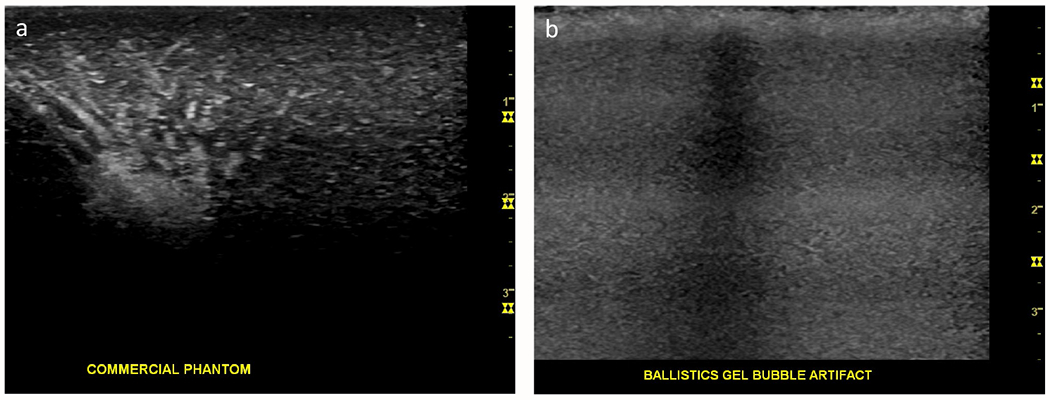
Illustration of the echogenic quality of a commercial phantom with numerous needle tracks obscuring a target (A) and a ballistics gel phantom with abundant air bubbles obscuring a target and needle path (B).
For overall teaching utility and impression, the 1:8 gelatin phantom scored the best with a median rating of 5, while the commercial phantom, ballistics gel, and 1:10 gelatin were also rated highly with a median score of 4 (Table 1, Figures 2C and D). Chicken breast and extra firm tofu were acceptable (median scores of 3), while again, canned cooked pork was felt to be the least useful for teaching. For overall ranking of the quality of the phantoms, the 1:8 gelatin model was the favorite, followed by the commercial phantom, 1:10 gelatin model, ballistics gel and extra firm tofu (tied), chicken breast, and finally canned cooked pork (Table 1, Figure 5).
Figure 5:
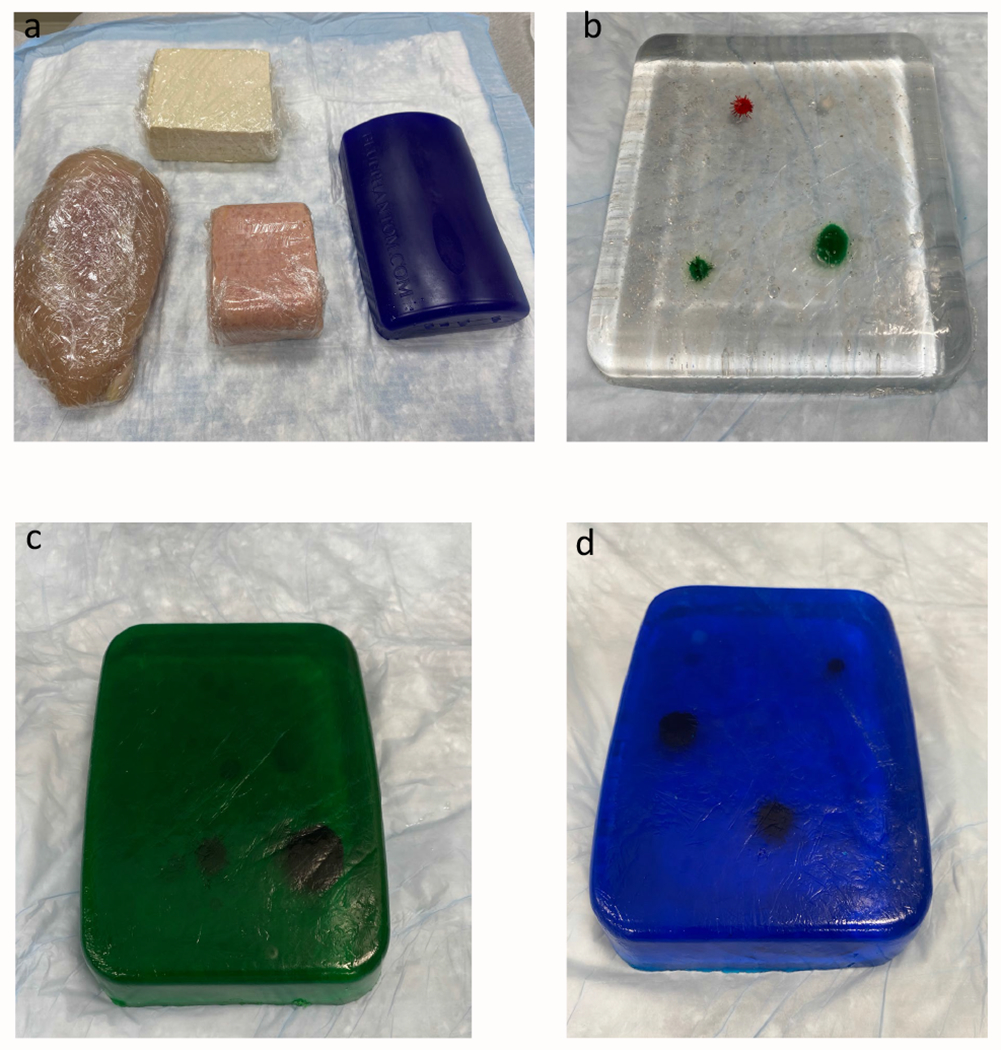
Homemade and commercial phantom models compared. A) From left, clockwise, commercial phantom, canned cooked pork, chicken breast, extra firm tofu. B) Ballistics gel phantom with craft pom poms as targets. C) Phantom made of 1:10 gelatin to water ratio with green food coloring, pitted olives, and cooked black beans. D) Phantom made of 1:8 gelatin to water ratio with blue food coloring, pitted olives, and cooked black beans.
Discussion
Ultrasound phantoms are excellent tools for teaching trainees how to scan for lesions, familiarizing them with fundamental fine needle aspiration biopsy technique and for increasing confidence prior to seeing their first patient. Commercial ultrasound phantoms are durable and shelf stable but expensive, and therefore may not be easily accessible. The Blue Phantom Soft Tissue Biopsy Ultrasound Training Block (CAE Healthcare, Sarasota, FL), which is what our training program uses, is currently listed at $59513. While one study found that the Blue Phantom demonstrated excellent durability even after 1,000 needle punctures14, the study fails to mention that the accumulation of track marks overtime limits the usefulness of the phantom as a training tool since it obscures the targets. Newer simulators such as the FioNA™ Fine Needle Aspiration Simulation Kit (Pacific Research Laboratories, Inc., Vashon Island, WA, USA), have replaceable skins and reusable bladders15 but has a current list price of $1,55916. In addition, the high price of these phantoms are cost prohibitive for use in workshops, where there are numerous trainees at one time, and in resource constrained settings6. This study did not assess the durability of the phantoms, but one study evaluating homemade models for vascular access found that the tofu model began degrading in under 20 pierces, while chicken breast, gelatin, and ballistics gel withstood more 25 piercings without losing structural integrity6. Moreover, the ballistics gel can be re-heated to remove track marks6.
Numerous homemade models have been described, which have several advantages to commercial phantoms, including accessibility of materials, cost, and ability to customize tissue consistency and targets17. In fact, phantoms for palpation-guided FNAB training using readily available materials, such as food items, have been used for many years2,18, but more recently, there has been an increased need for phantoms optimized for USFNA training given the increase in image-guided FNAB. The change in practice created a need for phantoms that are opaque (i.e. phantoms in which the targets cannot be seen with the naked eye), but in which the needle and target are reliably and well-visualized by ultrasound.
However, there is a remarkable variation in the materials and protocols used for fabricating phantoms. Studies have reported tofu12,19, gelatin , agar22, Premisorb23, silicone7, canned cooked pork11, ballistics gel9,24, and chicken breast10. Moreover, most of these phantoms are optimized for vascular access training and anesthesia-related interventions. The methods, advantages, and disadvantages for select homemade phantoms are summarized in table 2. Therefore, in this study we aimed to compare several low-cost, simple homemade phantoms tailored for ultrasound-guided fine needle aspiration biopsy practice. For example, several protocols using gelatin suggest using powdered psyllium seed husks or corn starch to improve echogenicity5,7,20, we chose not to include this step to simplify the protocol as much as possible to increase accessibility.
Table 2.
Summary of Selected Homemade Phantom Recipes
| Phantom type | Abbreviated recipe* | Advantages | Disadvantages |
|---|---|---|---|
| Gelatin | Mix 1 g gelatin per 8 or 10ml boiling water (23.6 or 29.5g gelatin per 1 cup water); add food coloring until opaque (optional); add targets; refrigerate until set (approximately 4 hours) | • Low cost • Easy and simple to construct • Excellent echogenicity • Good haptic quality |
• Requires refrigeration to construct and to extend shelf life • Moderate durability • Moderate shelf life |
| Gelatin7 |
Mix 20 g gelatin 40 g glycerin, 40 g sorbitol, and 20 g psyllium fiber per 1 cup boiling water; add targets; refrigerate until set (minimum 6 hours) |
• Low cost • Easy and simple to construct • Excellent echogenicity • Good haptic quality • Psyllium fiber adds echogenicity. Amount of psyllium can be adjusted based on preference • Sorbitol and glycerin increase flexibility and tear-resistance |
• Requires refrigeration to construct and to extend shelf life • Moderate durability • Moderate shelf life • Sorbitol and glycerin are not readily available at grocery stores |
| Gelatin21 | Add targets to mold; mix 40g gelatin, 20 grams psyllium fiber, 1 tablespoon citric acid, and 1 teaspoon blue food coloring per 500 ml boiling water; add targets; refrigerate overnight | • Low cost • Easy and simple to construct • Excellent echogenicity • Good haptic quality • Citric acid is a preserving agent (optional) |
• Requires refrigeration to construct and to extend shelf life • Moderate durability • Moderate shelf life |
| Gelatin20 | Add 28g gelatin to 250 ml warm water; microwave for 20 seconds; add 10 g cornstarch dissolved in 50ml warm water; add targets | • Low cost • Easy and simple to construct • Excellent echogenicity • Good haptic quality • Cornstarch adds echogenicity. Amount of cornstarch can be adjusted based on preference. |
• Requires refrigeration to construct and to extend shelf life • Moderate durability • Moderate shelf life |
| Gelatin5 |
Prepare 2 layers. Layer 1: Mix 21 g gelatin, 2 tablespoons psyllium fiber, and 10 drops of food coloring per 1 cup water; add targets; refrigerate for 2 hours. Layer 2: Mix 21 g gelatin, 1 tablespoon psyllium fiber, and 10 drops of food coloring per 1 cup water; let mixture cool for 15 minutes; pour mixture on top of layer 1; refrigerate overnight |
• Low cost • Easy and simple to construct • Excellent echogenicity • Good haptic quality • Allows for 2 separate layers with different degrees of echogenicity • Can also ensure targets are in center of mold if targets are added after layer 1 is set |
• Requires refrigeration to construct and to extend shelf life • Moderate durability • Moderate shelf life |
| Silicone7 | Mix 400 g Part A and 400g Part B of PlatSil Gel, 1200g Smith’s Theatrical Prosthetic Deadener, and a sprinkle of psyllim fiber; add targets; allow to set for minimum 4 hours | • Indefinite shelf life at room temperature • Highly durable |
• More expensive than ballistics gel and food-based phantoms • Materials must be purchased at specialty stores or websites |
| Ballistics gel9 | Cut ballistics gel block into 1-inch by 1-inch cubes; heat in oven set at 260°F (127°C) in oven-safe mold until melted completely; add targets; heat for another 30 minutes; allow gel to cool at room temperature for 2 hours |
• Indefinite shelf life at room temperature • Highly durable • Good echogenicity • Can be re-heated for re-use |
• Relatively more expensive than food-based phantoms • More difficult to construct (easy to introduce bubbles) • Moderate haptic quality |
| Ballistics gel24 | Break ballistics gel block into pieces; heat gel on stovetop over low heat until melted (approximately 200°F/93.3°C); add targets; heat additional gel to pour over targets | • Indefinite shelf life at room temperature • Highly durable • Good echogenicity • Can be re-heated for re-use • Stovetop melts gel faster than oven |
• Relatively more expensive than food-based phantoms • More difficult to construct (easy to introduce bubbles) • Moderate haptic quality Requires increased manipulation of hot gel |
| Agar22 | Mix 38 g of 900 g/cm2 agar to 750 ml water and bring to boil; add 1 teaspoon flour; pour half of mixture into mold and let set at room temperature for 20 minutes; add targets; add remainder of mixture as cap later; let set at room temperature for 20 minutes |
• Low cost • Simple and quick to construct • Does not require refrigeration • Highly durable • Can be re-heated for re-use • Flour adds echogenicity. Amount of flour can be adjusted based on preference |
• Moderate shelf life • Materials may only be available in specialty stores or websites |
| Premisorb23 | Insert foam targets into an empty 500ml IV fluid bag; fill with 500ml tap water and 15g of Premisorb; add food coloring (optional); seal IV fluid bag |
• Low cost • Simple and quick to construct • Semisolid material seals needle tracks. • Target able to be moved to different positions • Nonperishable |
• Materials not readily available outside operating room |
| Chicken breast | Use a knife to make a small incision into side of chicken breast; add targets into incision sites; wrap tightly in plastic wrap |
• Low cost • Simple and quick to construct • Good haptic quality • Good echogenic quality • Good structural integrity |
• Short shelf life • Requires special handling/risk of foodborne pathogens |
| Chicken breast10 | Add targets to surface of the chicken breast; either sandwich targets between another chicken breast or roll up chicken breast; wrap tightly in plastic wrap | • Low cost • Simple and quick to construct • Good haptic quality • Good echogenic quality • Good structural integrity |
• Short shelf life • Requires special handling/risk of foodborne pathogens |
| Canned cooked pork11 | Low cost Simple and quick to construct |
• Poor haptic quality • Poor echogenicity • Short shelf life • Poor durability |
|
| Tofu6–12,19 | • Low cost • Simple and quick to construct • Good echogenic quality |
• Moderate haptic quality • Short shelf life • Moderate durability |
Reported target options include: Craft pom poms, sponge, foam, balloons and gloves filled with water or gelatin, latex tubing or drinking straws filled with water, wooden dowels, needles, and food items such as pitted olives, various cooked beans, lentils, raisins, gummy bears, raisins, grapes, cherry tomatoes, hot dogs, blueberries.
In this study, the performance and teaching utility of the gelatin phantoms were similar to or better than the commercial phantom, with the 1:8 gelatin model performing better than the 1:10 gelatin model. They both demonstrated excellent haptic and echogenic qualities and were felt to be useful for practicing USFNA. In addition, the opacity of the gelatin models could be customized to the trainee’s experience. When transparent, the gelatin allowed for direct visualization of the targets, permitting easy correlation of hand placement and ultrasound images. Opacity could be increased when preparing the gelatin phantoms by adding more food coloring. In our experience, the gelatin phantoms can be stored at 4 degrees Celsius for at least 1 week, while chicken breast, tofu and canned cooked pork have the shortest shelf life and can be used for only a few days, and needed to be stored in a refrigerator if kept overnight. While the limited shelf life of the gelatin and other food-based phantoms may be perceived as a disadvantage, the phantoms are easy to make, inexpensive and are disposed of before track marks accumulate. Considering that the phantoms are mostly needed at the beginning stages of training and for a relatively short period of time, this disadvantage may not significantly reduce the usefulness of these phantoms for USFNA training. For those looking for a more durable option, ballistics gel was a good alternative to the commercial phantom. However, the participants in this study felt that both ballistics gel and commercial phantoms provided too much resistance in comparison to real human tissue, and that the visualization on ultrasound of the targets in the ballistics gel phantom was modest and slightly worse than the other phantoms, except for canned cooked pork. However, participants appreciated the long shelf life of the ballistics gel and that could be reheated to eliminate or reduce the number of track marks, extending its shelf life even further. Of note, the ballistic gel model was transparent, which made it useful in the early stages of training, but the opacity could not be adjusted since food coloring could not be added and mixed in since the material was so viscous.
Overall, if the phantoms can be made at least several hours prior to use, then gelatin phantoms are an excellent alternative to commercial phantoms. If a long shelf life and reusability are a priority, then ballistics gel may be the best option for a homemade phantom. Chicken breast and tofu are also great and inexpensive options if the phantoms are only needed for a brief period of time, such as at a workshop, and can be made only minutes ahead of time. Tofu in particular eliminates the need for special handling and potential exposure to foodborne illness. Canned cooked pork is not recommended for USFNA practice.
There are no size recommendations for ultrasound phantoms. Instead, we tried to create homemade phantoms that were similar in size to the commercial phantoms (17cm x13cm x 6cm). Extra firm tofu and canned cooked pork phantoms came in fixed sizes, but were a minimum of 5cm (2 inches) in depth. However, in our practice we generally use 1 inch (2.5 cm) 23 gauge needles. In some practices, 1.5 inch (3.8 cm) needles are used. We therefore recommend a minimum depth of 5cm (2 inches), and as the reviewer suggested, that at a minimum, the length and width should just be longer than the probe’s longest extent.
There are several limitations to this study. First, because of inherent differences in the properties of the materials, the same targets could not be used for all the phantoms. For example, the tofu and canned cooked pork phantoms could not accommodate larger targets such as olives, while craft pom poms were used for the ballistics gel because of their long-term shelf stability. In addition, the participants could not be blinded while testing the phantoms which may introduce potential bias in their assessments. Another limitation is that this study did not systematically evaluate the durability over time, mainly because the food-based phantoms had short shelf lives. It is therefore unclear how the ballistics gel phantom directly compares to the commercial phantom in terms of number of punctures it can sustain and retention of track marks. Cost was also imprecise and will likely vary depending on region and where the materials are purchased. In our study, the local supermarket prices were used, but we recognize that the study took place in a high cost of living setting.
In conclusion, depending on their intended use, most homemade phantoms are inexpensive and excellent substitutes for commercial phantoms and are an important educational tool that allow trainees to practice USFNA in a controlled environment.
Acknowledgments:
We thank Drs. Jean-Marc Cohen, Brie Kezlarian, Xiao-Jun Wei, Mega Lahori, Amir Dehghani, Darren Buonocore, and David Kim for their participation in this study. We thank Dr. Ronald Balassanian for providing a gelatin phantom recipe and inspiring this study.
Funding:
DLN receives support from the National Cancer Institute under award number K08CA263299. Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Cancer Institute under award number P30CA008748.
Footnotes
Declarations of interest: None.
References
- 1.Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer. 2001;93(4):263–268. doi: 10.1002/cncr.9040 [DOI] [PubMed] [Google Scholar]
- 2.Yang SR, Ljung BM. Teaching and learning FNA biopsy: An update for the modern audience. Cancer Cytopathology. 2019;127(10):615–617. doi: 10.1002/cncy.22169 [DOI] [PubMed] [Google Scholar]
- 3.Lèguevaque P, Motton S, Courbon F, Ricard M, Berry I, Querleu D. Evaluation of a Trainer Phantom in the Learning Phase of Sentinel Lymph Node Identification in Breast Cancer. World J Surg. 2011;35(5):995–1001. doi: 10.1007/s00268-011-0997-7 [DOI] [PubMed] [Google Scholar]
- 4.Li JW, Karmakar MK, Li X, Kwok WH, Kee WDN. Gelatin-Agar Lumbosacral Spine Phantom. Journal of Ultrasound in Medicine. 2011;30(2):263–272. doi: 10.7863/jum.2011.30.2.263 [DOI] [PubMed] [Google Scholar]
- 5.Richardson C, Bernard S, Dinh VA. A Cost-effective, Gelatin-Based Phantom Model for Learning Ultrasound-Guided Fine-Needle Aspiration Procedures of the Head and Neck. J Ultrasound Med. 2015;34(8):1479–1484. doi: 10.7863/ultra.34.8.1479 [DOI] [PubMed] [Google Scholar]
- 6.Selame LA, Risler Z, Zakaria SJ, et al. A comparison of homemade vascular access ultrasound phantom models for peripheral intravenous catheter insertion. J Vasc Access. 2021;22(6):891–897. doi: 10.1177/1129729820961941 [DOI] [PubMed] [Google Scholar]
- 7.Jug R, Jiang X “Sara.” Creating Custom Phantoms for Ultrasound-Guided Fine-Needle Aspiration Biopsy Training. AJSP: Reviews & Reports. 2018;23(4):176–179. doi: 10.1097/PCR.0000000000000254 [DOI] [Google Scholar]
- 8.Kebe Radulović M, Vivoda Tomšič M, Cimerman D, Gutnik H, Strojan Fležar M. A novel combined animal tissue model for freehand and ultrasound-guided fine needle aspiration biopsy and smear preparation techniques training. Diagnostic Cytopathology. 2021;49(1):39–45. doi: 10.1002/dc.24578 [DOI] [PubMed] [Google Scholar]
- 9.Amini R, Kartchner JZ, Stolz LA, Biffar D, Hamilton AJ, Adhikari S. A novel and inexpensive ballistic gel phantom for ultrasound training. World J Emerg Med. 2015;6(3):225–228. doi: 10.5847/wjem.j.1920-8642.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rippey JCR, Blanco P, Carr PJ. An Affordable and Easily Constructed Model for Training in Ultrasound-guided Vascular Access. J Vasc Access. 2015;16(5):422–427. doi: 10.5301/jva.5000384 [DOI] [PubMed] [Google Scholar]
- 11.Nolting L, Hunt P, Cook T, Douglas B. An Inexpensive and Easy Ultrasound Phantom: A Novel Use for SPAM. J Ultrasound Med. 2016;35(4):819–822. doi: 10.7863/ultra.14.06023 [DOI] [PubMed] [Google Scholar]
- 12.Zhang YF, Li H, Wang XM. Technical Report: A Cost-Effective, Easily Available Tofu Model for Training Residents in Ultrasound-Guided Fine Needle Thyroid Nodule Targeting Punctures. Korean J Radiol. 2019;20(1):166–170. doi: 10.3348/kjr.2017.0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trainers MS. Soft Tissue Biopsy Ultrasound Training Block - Medical Skills Trainers. Accessed December 20, 2022. https://medicalskillstrainers.cae.com/soft-tissue-biopsy-ultrasound-training-block-/p
- 14.Schofer JM, Nomura JT, Bauman MJ, Sierzenski PR. Prospective durability testing of a vascular access phantom. West J Emerg Med. 2010;11(4):302–305. [PMC free article] [PubMed] [Google Scholar]
- 15.Alcaraz-Mateos E, Jiang XS, Mohammed AAR, et al. A novel simulator model and standardized assessment tools for fine needle aspiration cytology training. Diagn Cytopathol. 2019;47(4):297–301. doi: 10.1002/dc.24105 [DOI] [PubMed] [Google Scholar]
- 16.FioNA Fine Needle Aspiration Simulation Kit. Accessed December 20, 2022. https://www.sawbones.com/fine-needle-fiona-model1940.html
- 17.Schwartz CM, Ivancic RJ, McDermott SM, Bahner DP. Designing a Low-Cost Thyroid Ultrasound Phantom for Medical Student Education. Ultrasound in Medicine & Biology. 2020;46(6):1545–1550. doi: 10.1016/j.ultrasmedbio.2020.01.033 [DOI] [PubMed] [Google Scholar]
- 18.Shidham VB, Varsegi GM, D’Amore K, Shidham A. Preparation and using phantom lesions to practice fine needle aspiration biopsies. J Vis Exp. 2009;(31):1404. doi: 10.3791/1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollard BA. New model for learning ultrasound-guided needle to target localization. Reg Anesth Pain Med. 2008;33(4):360–362. doi: 10.1016/j.rapm.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 20.Hakimi AA, Armstrong WB. Improving on the Do-It-Yourself Ultrasound-Guided Fine-Needle Aspiration Simulation Phantom. Journal of Ultrasound in Medicine. 2021;40(4):815–819. doi: 10.1002/jum.15461 [DOI] [PubMed] [Google Scholar]
- 21.How To: DIY Ultrasound Guided Peripheral IV Phantom - UCSD Ultrasound. Accessed March 1, 2023. https://emultrasound.sdsc.edu/index.php/2017/12/07/usgpiv-phantom/
- 22.Earle M, Portu GD, DeVos E. Agar ultrasound phantoms for low-cost training without refrigeration. Afr J Emerg Med. 2016;6(1):18–23. doi: 10.1016/j.afjem.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Glass NL, Power RW. Technical communication: new teaching model for practicing ultrasound-guided regional anesthesia techniques: no perishable food products! Anesth Analg. 2010;110(4):1233–1235. doi: 10.1213/ANE.0b013e3181cc558b [DOI] [PubMed] [Google Scholar]
- 24.Morrow DS, Broder J. Cost-effective, Reusable, Leak-resistant Ultrasound-guided Vascular Access Trainer. J Emerg Med. 2015;49(3):313–317. doi: 10.1016/j.jemermed.2015.04.005 [DOI] [PubMed] [Google Scholar]


