Abstract
Introduction:
Patients who discontinue methadone for opioid use disorder are at increased risk of overdose and death. We know little about how patients make the decision to stop treatment. This study explored reasons why patients discontinue methadone treatment.
Methods:
We conducted 20 individual semi-structured patient interviews and two staff focus groups, each with five participants, at two opioid treatment programs in Baltimore, MD, in the United States from June 2021 to May 2022. Patient interviews and staff focus groups covered three domains: 1) reasons why patients may want to discontinue methadone; 2) perspectives about the ideal length of methadone treatment; and 3) changes that could improve retention. We used a modified grounded theory approach to code interviews, identify emergent themes, and develop a conceptual model.
Results:
We identified three themes related to patients’ internal relationships to methadone: patients (1) viewed methadone as a bridge to opioid-free recovery, (2) believed that long-term methadone damages the body, and (3) felt that methadone increases craving for cocaine; and three themes related to their external relationships with opioid treatment programs and society at large: patients (4) viewed daily dosing as burdensome, (5) feared methadone inaccessibility could trigger relapse, and (6) experienced stigma from friends, family, and peers. Patients with internal reasons planned to stop as soon as possible and asked for education about perceived side effects and treatment for cocaine craving to promote retention. Patients with external reasons were willing to continue for longer and asked for adaptive take-home policies and reduced societal stigma around methadone.
Conclusions:
Patients want to discontinue methadone either because of their internal relationship to methadone and its real or perceived side effects, or because of their external experiences with opioid treatment programs and societal stigma of methadone. To improve retention, clinical and policy changes should consider responses to both of these categories of reasons.
Keywords: opioid use disorder, methadone, retention, opioid agonist treatment, opioid treatment program
1. Introduction
Less than 15% of the more than 2.7 million individuals in the United States with opioid use disorder (OUD) received methadone, buprenorphine, or extended-release naltrexone in 2020 (Krawczyk, Rivera, et al., 2022; Substance Abuse and Mental Health Services Administration., 2021a), despite evidence that at least 6–12 months of treatment with one of these medications is the standard of care (Crotty et al., 2020; National Academies of Sciences, Engineering, and Medicine 2019, 2019; National Institute on Drug Abuse, 2012). This treatment gap reflects both a failure to initiate care and to retain patients in treatment (Williams et al., 2019). Of the three FDA-approved medications for OUD, methadone has the best retention in care (Amato et al., 2005; Hser et al., 2014). However, even methadone treatment retention remains poor and highly variable, with 6-12-month retention rates in methadone treatment ranging from 40% to 65% in systematic reviews (O’Connor et al., 2020; Timko et al., 2016). Early methadone discontinuation can have profound consequences: all-cause and overdose mortality increase by 23 and 10 deaths per 1000 person-years, respectively, for individuals who stop methadone treatment (Sordo et al., 2017).
Within the United States, methadone for OUD must be dispensed directly from state and federally licensed opioid treatment programs (OTPs) (Substance Abuse and Mental Health Services Administration., 2021b). Historically, patients were required to visit an OTP at least 5–6 days a week for observed dosing during the first 90 days of treatment (Crotty et al., 2020; Substance Abuse and Mental Health Services Administration, 2021b), after which they could receive more frequent take-home doses. In response to the COVID-19 pandemic, federal guidance from 2020 temporarily allowed OTPs flexibility for take-home doses earlier in treatment (Levander et al., 2021, 2022). Despite the safety (Jones et al., 2022) and improved retention (Hoffman et al., 2022) associated with this dosing flexibility, many OTPs did not implement these changes broadly (Brothers et al., 2021; Krawczyk, Maniates, et al., 2022). Most recently, in December 2022 the Substance Abuse and Mental Health Services Administration (SAMHSA) proposed to make permanent these and other changes to methadone treatment of OUD (Substance Abuse and Mental Health Services Administration, 2022).
As these changes are debated, it is crucial to understand patients’ experience of methadone and how that experience shapes their intentions about continuing treatment. Prior studies have shown that older age (Darker et al., 2016), sufficient or higher doses of methadone (Amato et al., 2005; Biondi, 2022), cessation of other drug use (Deck & Carlson, 2005; Proctor et al., 2015), and stable housing (Deck & Carlson, 2005; Huissoud et al., 2012) are all associated with improved retention. However we know less about how patients, themselves, make the decision to continue or discontinue medications for OUD. Qualitative studies conducted more than a decade ago or among exclusively White patients found that patients cited conflicts with program, high clinic fees, incarceration, and stigma as reasons for discontinuing methadone (Randall-Kosich et al., 2020; Reisinger et al., 2009). Recently, the spread of fentanyl and other high-potency synthetic opioids has dramatically increased overdose risk, especially for Black, American Indian/Alaska Native, and Hispanic or Latino individuals (Friedman & Hansen, 2022). In this context, we must assess reasons for discontinuation from a diverse cohort of individuals actively enrolled in methadone during the fentanyl era and explore interventions that could promote retention among these patients.
The goal of this study was to characterize how patients in the first year of methadone treatment for OUD decide whether and when to discontinue methadone. We conducted qualitative analyses of individual patient interviews and OTP staff focus groups to uncover shared and distinct themes from patients and clinicians. We explored three related domains: 1) reasons why patients previously, currently, or perhaps in the future would want to discontinue methadone; 2) how these reasons inform patient perspectives about the ideal length of treatment on methadone; and 3) perspectives on clinical or policy changes to promote retention in treatment.
2. Methods
2.1. Setting & study population
We obtained a convenience sample of patients and clinicians from two OTPs in Baltimore, Maryland, a city where 58% of the population identifies as Black of African American, 28% as White, and 8% Hispanic or Latino (Baltimore City Department of Planning, 2021). One OTP is a comprehensive, community-based outpatient substance use recovery program with an approximate census of 750 patients. The second is a comprehensive outpatient drug addiction treatment center associated with a university medical center with a census of about 120 patients.
Patients had to meet the following eligibility criteria: (1) age 18 years or older, (2) able to give verbal informed consent and to conduct an interview in English, (3) access to Zoom videoconferencing either at their OTP or through a personal device, (4) active enrolment in methadone treatment at one of the two study site OTPs, and (5) in the first 12 months of continuous methadone treatment. Patients could have started their current methadone treatment episode at a different OTP. We enrolled patients independent of whether they expressed a desire to discontinue methadone. Clinic staff had to be employed by one of the two study site OTPs as a counselor, therapist, peer support specialist, nurse, nurse practitioner, physician assistant, or physician.
We recruited OTP staff through presentations at virtual all-staff meetings and through emails to OTP staff. We recruited patient participants through flyers and through direct referral from counselors. One author (APT) screened potential participants to verify eligibility and to describe the study procedures.
2.2. Data collection
Three of the authors (APT, JDP, and GC) collaboratively developed the individual interview guide and focus group discussion guide to explore reasons for discontinuing methadone treatment (see Supplement for interview and discussion guides). For patients who actively wished to discontinue, the interview guide focused on current reasons. For others, the guide focused on reasons for discontinuing prior episodes of methadone treatment, or, for those without prior treatment episodes, on reasons they might discontinue in the future. The individual interview guide was pilot tested with two patients and five clinician-researchers and subsequently revised.
One author (APT) conducted all individual patient interviews and staff focus groups from June 2021 to April 2022 using Zoom, version 5.11.1 without any non-participants present. Each interview and focus group lasted approximately 60 minutes. All interview and focus group participants provided verbal informed consent and received a $30 gift card honorarium for their time. An institutional review board at Johns Hopkins University approved this research. We followed the Consolidated Criteria for Reporting Qualitative Research (COREQ) reporting guideline (Tong et al., 2007).
2.3. Data analysis
Interviews and focus groups were audio-recorded and professionally transcribed. Following a modified grounded theory approach (Foley & Timonen, 2015), two authors (APT & JDP, both board-certified addiction medicine physicians) with training in qualitative methods reviewed the two focus groups and first four individual interview transcripts to develop initial codes. Three authors (APT, JDP, and GC) reviewed the preliminary codes for clarity and consistency between coders and developed an initial codebook. APT & JDP completed a second round of coding for the initial transcripts using the codebook, then coded subsequent individual interviews independently and in parallel with regular meetings to iteratively revise codes, update the codebook, and discuss emerging themes. APT & JDP supplemented analysis with analytic memos to explore the relationship among codes and to aid in the development of a conceptual model with input from all study authors. Individual interviews were conducted concurrently until we reached thematic saturation. We used NVivo qualitative software to organize codes and facilitate analysis.
3. Results
Thirty-five patients were referred to participate in this study. Eight of these patients did not meet inclusion criteria: four had been enrolled in methadone for more than 12 months in the current treatment episode, three were enrolled in a nonparticipating OTP, and one was enrolled in buprenorphine treatment. Six individuals were unable to be scheduled for interviews despite three attempts. One individual interview was not completed because thematic saturation had been reached. Six staff members from each OTP expressed interest in joining a focus group, but only five joined from each at the scheduled times. One individual from the second OTP did not have a functioning video or microphone but listened, watched, and contributed via a text-based chat that was added to the second focus group transcript.
3.1. Participant characteristics
The median patient interview length was 43 minutes (interquartile range [IQR] 40.3–46.3). Among the 20 patient participants, the median age was 45 years (IQR 37–53); 7 (35%) identified as female; 10 (50%) identified as Black, one (8%) as Native American, and nine (45%) as White; one (5%) identified as Hispanic/Latino (Table 1). The median time enrolled in the current methadone treatment episode was 4.2 months (IQR 1.9–5.0), median methadone dose was 70mg (IQR 53.5–87.5), and median take-homes per week was 2 (IQR 2–4). Eight (40%), were enrolled in their first methadone treatment episode and had never previously discontinued. Among the 10 staff members in the two focus groups, median age decile was 50–59 years; 7 (79%) identified as female; 4 (40%) and identified as Black and 6 (60%) as White. Seven (70%) of the focus group participants were counselors or therapists and the remainder comprised a peer support specialist, a nurse, and a nurse practitioner. These participants reported median time working at the specific study OTP as 5.5 years (IQR 3.5, 9.8).
Table 1:
Participant Characteristics
| Patients (n=20) |
Staff (n=10) | ||
|---|---|---|---|
| Age, median years (IQR) or median decile | 45 (37, 53) | 50–59 | |
| Female, n (%) | 7 (35) | 7 (79) | |
| Race, n (%) | |||
| Black/African American | 10 (50) | 4 (40) | |
| Native American | 1 (5) | 0 | |
| White | 9 (45) | 6 (60) | |
| Hispanic/Latinx, n (%) | 1 (5) | 0 | |
| Clinic, n (%) | |||
| Clinic A | 8 (40) | 6 (60) | |
| Clinic B | 12 (60) | 4 (40) | |
| Length of current methadone treatment episode, median months (IQR) | 4.2 (1.9, 5.0) | - | |
| Methadone total daily dose, mg (IQR) | 70 (53.5, 87.5) | - | |
| Methadone take-homes per week (IQR)* | 2 (2, 4) | - | |
| Number of prior methadone treatment episodes, n (%) | |||
| 0 | 8 (40) | - | |
| 1–3 | 10 (50) | - | |
| ≥4 | 2 (10) | - | |
| Opioid(s) used most often prior to starting methadone, n (%) | |||
| Heroin/fentanyl | 19 (95) | - | |
| Oxycodone | 1 (5) | - | |
| Injection drug use prior to starting methadone, n (%) | 11 (55) | - | |
| Years working at specific opioid treatment program, median (IQR) | - | 5.5 (3.5, 9.75) | |
| Clinical role, n (%) | |||
| Counselor/therapist | - | 7 (70) | |
| Peer Support Specialist | - | 1 (10) | |
| Nurse | - | 1 (10) | |
| Nurse Practitioner | - | 1 (10) | |
Excluding two participants who had methadone delivered to their residential treatment centers every 14 days
3.2. Reasons for discontinuing methadone
Patient interviews and staff focus groups revealed two categories of themes regarding why patients wanted to stop methadone treatment (Figure 1). Three themes related to patients’ internal relationship to methadone and three themes related to patients’ external relationship with OTPs and society at large. Each of these two theme categories informed one of two orientations about the ideal length of treatment: patients with internal reasons for discontinuing wanted to stop methadone as quickly as possible; those with external reasons were willing to continue as long as the benefits outweighed the burdens. These orientations, meanwhile, aligned with different types of changes that patients and staff believed would support retention.
Fig. 1.
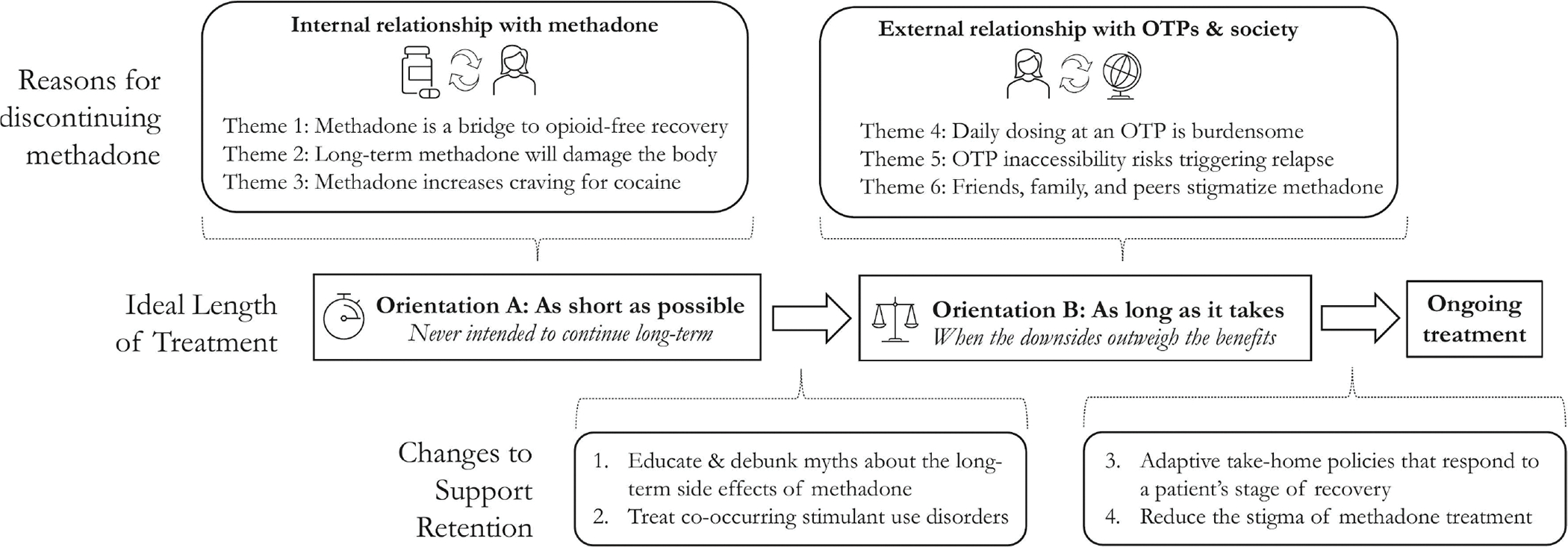
Conceptual Model of Why Patients Stop Methadone Treatment for Opioid Use Disorder.
Abbreviations: OTP, opioid treatment program.
We assigned all patients pseudonyms to protect anonymity. For additional context, we also list each patient’s total daily dose of methadone and number of take-homes per week.
3.2.1. Internal reasons for discontinuing methadone
For some patients, the primary reason to stop methadone stemmed from their personal relationship with methadone, itself. We labeled these “internal reasons.”
Theme 1: Methadone is a bridge to opioid-free recovery.
Some patients conceptualized methadone as a temporary bridge to opioid-free recovery, not as indefinite treatment for a chronic medical condition. For Anthony (40s, male, 50mg, methadone delivered every two weeks to his residential treatment program), methadone was “just a crutch, though, because you’re not completely clean…That’s what methadone is, it’s a means to an end. But the end should not last forever…The end is getting off methadone.” An OTP staff member agreed, telling us “some do not feel it is ‘real recovery’ because it is not abstinence.” These patients defined recovery as being opioid-free and never intended to continue methadone long-term. For them, discontinuing methadone was a sign of success.
Theme 2: Long-term methadone damages the body
A second theme for why patients discontinue was the belief that long-term methadone would have harmful side effects. David (50s, male, 50mg, two take-homes per week) thought about stopping methadone “every day” because of these concerns:
I don’t want to be on methadone because I see how it do people’s bodies and how it destroys your bones and stuff, and I don’t want to get to the point where I can’t walk around, or I’m leaning when I walk, bending over and stuff…They say it destroys your teeth.
OTP staff also raised these patient concerns, with one saying she frequently hears patients telling her “false information about losing their teeth, their bones get brittle.” For Elizabeth (30s, female, 110m, two take-homes per week), these concerns were so prominent that they were the first thing she mentioned when asked how she would describe methadone to a friend: “I would tell her it’s something good to try but not for long ‘cause it bothers your teeth and mess with your bones.”
Theme 3: Methadone increases craving for cocaine
The last theme was how methadone increased craving for cocaine. Susan (40s, female, 57mg, two take-homes per week) previously discontinued methadone because of these cravings. At the time of the interview, she was “scared to be on [methadone] a long time” because “it gives me a stronger urge to want to smoke.” OTP staff members in the first focus group also noted that patient’s report increased cocaine cravings from methadone.
3.2.2. Orientation A: “As short as possible”
Patients with these internal reasons for stopping methadone described their ideal length of methadone treatment as some variation of “as short as possible” (see Figure 1). For Mary (50s, female, methadone delivered every 14 days to her residential treatment program), “if you could just get off methadone as quickly as you could get on it, 90 days would be good.” Anthony, the man who described methadone as a crutch, wanted to start tapering off methadone as soon as he left his residential drug treatment program: “once I get done that program, I hope to be moving off of the methadone program and exiting off.” An OTP staff member saw this orientation often: “I’ve been in this field over 15 years…it’s usually like a short-term goal of 1, 2, 3 months to a year.”
Some patients with this orientation said that nothing could change their plan to stop methadone treatment. When asked about what could change about methadone to help her stay on it longer, Elizabeth (30s, female, 85mg, two take-homes per week) said, “Nothing. I just don’t want to be on it long.” Others needed their specific internal reason addressed. Some patients concerned about damage to their bones or teeth said that education could help:
Barbara (30s, female, 90mg, two take-homes per week): I guess just more information about it, I guess. More facts about the side effects, and like all of the good and the bad; Just being more knowledgeable about everything about it.
Another patient had heard concerns about bone damage but had changed his mind after trying methadone and after talking to her doctor:
Gary (50s, male, 70mg, six take-homes per week): Yeah, I heard all kinds of crazy stuff…but I changed my mind totally…Because now I experienced it…The doctor explained everything to me about methadone…He said it was all a myth. Just all hearsay…Everything he said made sense.
Finally, patients with cocaine craving triggered by methadone asked for treatment for cocaine use disorder. Susan, the woman who described cocaine craving, would continue methadone indefinitely “if you could take a pill and it would block you from getting high from opioids and cocaine…it takes away those cravings.”
3.2.3. External reasons for discontinuing methadone
A second group of patients wanted to discontinue methadone because of reasons related to the experience and stigma of being enrolled in an OTP for methadone. We labeled these “external reasons.”
Theme 4: Daily dosing at an OTP is burdensome
One theme that emerged was that daily dispensed dosing at an OTP is burdensome because of transportation challenges or travel restrictions. Without access to a car, patients had to walk, take a bus, or pay for a taxi or ride-share daily. This introduced major challenges to their daily lives. Barbara (30s, female, 70mg, four take-homes per week) wanted to stop within the next six months because “I’m not mobile right now, so I don’t have a vehicle. So I’m walking two miles each way, and that’s a lot.” OTP staff also identified daily dosing and transportation challenges as reasons why patients discontinue. One staff member said “they might say ‘we don’t have no money for no bus fare or for no bus pass’ and ‘I live maybe 30 or 40 minutes away.’” Other patients felt constrained because they felt they could not travel while enrolled in an OTP. Michael (30s, male, 80mg, two take-homes per week) discontinued methadone previously because he had to choose between his job and methadone:
I was traveling for the job, which made me miss multiple days. So I would have to be reinstated over and over and over. I would have to do it every week…So it just didn’t work out.
Theme 5: OTP inaccessibility risks triggering relapse
Separate from the burden of obtaining care, a theme emerged of how some patients, like George (50s, male, 95mg, six take-homes per week), live in a state of precarity while enrolled in methadone treatment. These patients wanted to stop methadone because they worried that their OTP might be inaccessible one day, and that the subsequent withdrawal would trigger relapse, since street opioids are always available:
George: There could be a bad snowstorm or something and I can’t get to methadone. Then I’m gonna start getting sick, and then if I can get heroin, which is realistic, rather than staying sick I’m gonna get that.
Barbara shared this concern in the context of the commute to her OTP “I’m walking 2 miles each way…what if I have a weak moment, or a weak day, or something, and I don’t feel like going? I have money, and I [could] decide to mess up.” For these patients, methadone from an OTP was harder to reach than illicit opioids; they believed that tapering off methadone was safer than risking a missed dose, which could trigger relapse. This idea of precarity was the only theme that arose in patient interviews, but not in staff focus groups. OTP staff from both focus groups noted that some patients suddenly discontinue without warning or explanation, with one staff member telling us that “it’s hard to tell why, because some will just drop out of treatment.” Patients, however, related sudden discontinuation back to the challenges of accessing their OTP on a nearly daily basis.
Theme 6: Friends, family, and peers stigmatize methadone
Patients and OTP staff members also pointed to stigma from friends, family, and peers in recovery as a reason for stopping methadone. This theme of pressure from patients’ social networks was exemplified by Jennifer (32, female, 85mg, two take-homes per week), who felt that her Narcotics Anonymous peer-recovery community “pushed me to try to get off [methadone] when I wasn’t ready to get off it” because “some of them think that you’re not clean if you’re on methadone.” Others felt devalued as members of their peer-recovery network because they could not publicly commemorate their time in recovery or serve as sponsors while enrolled in methadone treatment. Pressure to stop methadone also came from family and friends, as noted by a staff member in the first focus group who said, “I hear families say that methadone is worse than heroin.”
3.2.4. Orientation B: “As long as it takes”
These external reasons for stopping methadone informed a second orientation toward the ideal length of methadone treatment: “as long as it takes” (see Figure 1). Patients with this orientation expected to stop at some, undetermined point in the future, once the burdens outweighed the benefits. They planned to stop methadone once they achieved stability, although they did not have explicit indicators of what stability would look like. As Susan (50s, female, 120mg, two take-homes per week) put it, “I hope I’ll just know.” Susan went on to say:
It would be a feeling. Depends on how I will feel that particular day. Like, “Man, I ain’t feeling this shit no more.” That’s how I would decide…Really, it’ll be in my feelings…I don’t know. Maybe one day I may get up and I might be tired of it, just say, “Man, forget it.”
Some patients with this orientation would be willing to continue methadone long-term if take-home policies adapted to their stage of recovery. Barbara, the woman walking two miles to her OPT, had been in methadone treatment for nine months and felt that her OUD had stabilized, but she worried that she would be unable to get a job because of the burdens of daily dosing at her OTP:
I’m looking for a job right now at the same time, and it’s going to be really difficult for me to get to the clinic, and then to get to work.…If I’m making all my appointments now, and I’m there, and my [urine drug tests] are showing that it’s metabolized in my system, I feel as though I should be able to get a week at a time.
Steven (30s, male, 80mg, two take-homes per week) was similarly frustrated with how long it took for him to transition to more take-homes, saying “if I had take-homes and then I had to come up there once, twice, maybe two times a week, I could see myself staying on it for longer”
Last, patients with this orientation felt that reducing the societal stigma and discrimination toward methadone would help them to remain in treatment.
Patricia (30s, female, 120mg, two take-homes per week): There’s people out there like, “Oh, you’re just substituting for another drug.” And it’s hard to explain to people that don’t have addiction.
Staff from both focus groups generally agreed that stigma reduction around methadone at a societal level would help to support long-term methadone retention. A staff member described how some patients are “afraid that if people at work are going to find out, they’re going to get fired.”
4. Discussion
This qualitative study of methadone patients and clinical staff revealed two categories of reasons that patients seek to stop methadone treatment: reasons related to patients’ internal relationship with methadone, and reasons related to their external relationships with opioid treatment programs and society at large. Patients who primarily cited internal reasons sought to discontinue methadone as fast as possible and requested education about perceived side effects and treatment of cocaine use disorder to improve retention. Patients with external reasons were willing to continue methadone until they reached stability and felt that retention would be improved by take-home policies that adapted to their stage of recovery and by reduced societal stigma around methadone treatment.
A novel finding from this study is that some patients never intended to continue methadone long-term because of internal reasons related to fear of bone or dental damage or to increased cocaine craving. Notably, patients did not cite concerns about weight gain or loss of libido, two known side effects of methadone (Substance Abuse and Mental Health Services Administration., 2021b, p. 63). The internal reasons they did cite present unique opportunities for improving retention on methadone treatment. Long-term exposure to any opioid is associated with low bone mineral density, especially among men and among individuals with heavy alcohol use (Ramli et al., 2021). However, many details about this association remain unclear, including its mechanism, timing of onset, and the relative risk of methadone compared to other opioids (Grey et al., 2011; Kim et al., 2006; Ramli et al., 2021). A paucity of studies has examined the effects of methadone on dental health (Brondani & Park, 2011); however opioids, in general, suppress salivary secretion (Titsas & Ferguson, 2002) and have been associated with xerostomia (Götrick et al., 2004) and an increase in sugar cravings (Carr & Papadouka, 1994; Zador et al., 1996).
Approximately 40–60% of individuals enrolled in methadone maintenance treatment have co-occurring cocaine use disorder (Dhingra et al., 2015; Leri et al., 2003; Sees et al., 2000). Prior work has demonstrated increased craving for cocaine while enrolled in methadone (McNeil et al., 2020); however, to our knowledge this is the first study to show that increased cocaine craving is sometimes the dominant reason for discontinuing methadone. Neither SAMHSA nor the American Society of Addiction list any of these three concerns (bone damage, dental damage, or cocaine craving) as side effects or topics to cover when counseling patients who are starting methadone (American Society of Addiction Medicine, 2020; Substance Abuse and Mental Health Services Administration., 2021b). Patients could perhaps benefit from education on what is known about these risks, treatment for these conditions, and more rigorous research into these associations.
The external reasons for discontinuing methadone treatment described here highlight the importance of structural changes to methadone treatment in the United States. For patients, dosing flexibility can be liberating (Harris et al., 2022; Suen et al., 2022), makes treatment more attractive (Deering et al., 2011), and increases treatment satisfaction while reducing stigma (Frank et al., 2021). SAMHSA’s proposed rule changes allow for take-home doses on treatment initiation and reduces the frequency of required visits for patients with unreliable transportation, among other changes (Substance Abuse and Mental Health Services Administration, 2022). In light of our findings here, this dosing flexibility—if implemented by OTPs—has the potential to improve retention.
This study has a number of limitations. First, the themes uncovered here may not generalize to patients in methadone treatment outside of Baltimore or to patients with more than 12 months in their current methadone treatment episode. Second, patient recruitment may have been biased by our requirement that patients use Zoom for a video interview. We did so to protect patients and staff during the COVID-19 pandemic. Third, we included patients whether or not they expressed an active desire to stop methadone treatment. For patients who did not actively wish to stop methadone treatment, we focused interviews on discontinuation from prior treatment episodes or on reasons they may perhaps discontinue in the future. These responses might have been subject to recall bias or they might represent theoretical, instead of actual, reasons for discontinuing. Fourth, this study was limited to patients in treatment for less than one year; reasons for discontinuation may change after this period. Last, interviews and focus groups exclusively asked about reasons for discontinuing methadone and not about reasons for continuing methadone.
Conclusion
In this qualitative study, opioid treatment program patients and staff described similar reasons that patients seek to discontinue methadone treatment for OUD. Some patients wanted to stop methadone as quickly as possible and were motivated by a preference for opioid-free recovery, fear of bone or dental damage, or increased cocaine craving. Addressing these internal reasons with education, treatment, and research may present new opportunities to improve retention in methadone treatment. Other patients were willing to continue methadone until the burdens outweighed the benefits. Patients with this orientation described reasons for stopping such as the burdens of daily dosing at an OTP; fear of relapse after missing a dose; and stigma from family, friends, and peers in recovery. These external reasons show how patients experience the challenges of existing systems of care for methadone treatment and reinforce the importance of structural changes to improve retention in methadone treatment.
Supplementary Material
Highlights.
We explored reasons why patients stop methadone for treatment of opioid use disorder.
Some patients prefer opioid-free recovery.
Some patients fear bone and dental damage or have increased cocaine craving.
Some patients find daily dosing too burdensome or face stigma from family and peers.
Patients asked for education about side effects and adaptive take-home policies.
Acknowledgements:
We gratefully acknowledge all study participants for sharing their experiences with us.
Funding:
This work was supported by the National Institute on Drug Abuse-funded Research in Addiction Medicine Scholars (RAMS) program (grant number R24DA033211).
Footnotes
Declaration of interests: Dr. Weiss has consulted to Alkermes. Dr. Stoller serves on the American Association for the Treatment of Opioid Dependence board and consults for Berkshire Biomedical.
CRediT authorship contribution statement
A. Thakrar: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Writing – Original Draft, Writing – Review & Editing, Visualization, Project Administration. J. Pytell: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision. K. Stoller: Resources, Writing – Review & Editing. V. Walters: Resources, Writing – Review & Editing. R. Weiss: Conceptualization, Methodology, Writing – Review & Editing. G. Chander: Conceptualization, Methodology, Resources, Writing – Review & Editing, Supervision, Project administration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Amato L, Davoli M, Aperucci C, Ferri M, Faggiano F, & Pmattick R (2005). An overview of systematic reviews of the effectiveness of opiate maintenance therapies: Available evidence to inform clinical practice and research. Journal of Substance Abuse Treatment, 28(4), 321–329. 10.1016/j.jsat.2005.02.007 [DOI] [PubMed] [Google Scholar]
- American Society of Addiction Medicine. (2020). The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. Journal of Addiction Medicine, 14(2S), 1–91. 10.1097/ADM.0000000000000633 [DOI] [PubMed] [Google Scholar]
- Baltimore City Department of Planning. (2021). Data & Demographics—2020 Census Data. Department of Planning. https://planning.baltimorecity.gov/planning-data [Google Scholar]
- Biondi BE (2022). Factors associated with retention on medications for opioid use disorder among a cohort of adults seeking treatment in the community. Clinical Practice, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondani M, & Park PE (2011). Methadone and Oral Health – A Brief Review. 85(2), 8. [PubMed] [Google Scholar]
- Brothers S, Viera A, & Heimer R (2021). Changes in methadone program practices and fatal methadone overdose rates in Connecticut during COVID-19. Journal of Substance Abuse Treatment, 131, 108449. 10.1016/j.jsat.2021.108449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, & Papadouka V (1994). The role of multiple opioid receptors in the potentiation of reward by food restriction. Brain Research, 639(2), 253–260. 10.1016/0006-8993(94)91738-8 [DOI] [PubMed] [Google Scholar]
- Crotty K, Freedman KI, & Kampman KM (2020). Executive Summary of the Focused Update of the ASAM National Practice Guideline for the Treatment of Opioid Use Disorder. Journal of Addiction Medicine, 14(2), 99–112. 10.1097/ADM.0000000000000635 [DOI] [PubMed] [Google Scholar]
- Darker CD, Ho J, Kelly G, Whiston L, & Barry J (2016). Demographic and clinical factors predicting retention in methadone maintenance: Results from an Irish cohort. Irish Journal of Medical Science, 185(2), 433–441. 10.1007/s11845-015-1314-5 [DOI] [PubMed] [Google Scholar]
- Deck D, & Carlson MJ (2005). Retention in publicly funded methadone maintenance treatment in two Western States. The Journal of Behavioral Health Services & Research, 32(1), 43–60. 10.1007/BF02287327 [DOI] [PubMed] [Google Scholar]
- Deering DEA, Sheridan J, Sellman JD, Adamson SJ, Pooley S, Robertson R, & Henderson C (2011). Consumer and treatment provider perspectives on reducing barriers to opioid substitution treatment and improving treatment attractiveness. Addictive Behaviors, 36(6), 636–642. 10.1016/j.addbeh.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Dhingra L, Perlman DC, Masson C, Chen J, McKnight C, Jordan AE, Wasser T, Portenoy RK, & Cheatle MD (2015). Longitudinal analysis of pain and illicit drug use behaviors in outpatients on methadone maintenance. Drug and Alcohol Dependence, 149, 285–289. 10.1016/j.drugalcdep.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley G, & Timonen V (2015). Using Grounded Theory Method to Capture and Analyze Health Care Experiences. Health Services Research, 50(4), 1195–1210. 10.1111/1475-6773.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Mateu-Gelabert P, Perlman DC, Walters SM, Curran L, & Guarino H (2021). “It’s like ‘liquid handcuffs”: The effects of take-home dosing policies on Methadone Maintenance Treatment (MMT) patients’ lives. Harm Reduction Journal, 18(1), 88. 10.1186/s12954-021-00535-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, & Hansen H (2022). Evaluation of Increases in Drug Overdose Mortality Rates in the US by Race and Ethnicity Before and During the COVID-19 Pandemic. JAMA Psychiatry, 79(4), 379–381. 10.1001/jamapsychiatry.2022.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götrick B, Akerman S, Ericson D, Torstenson R, & Tobin G (2004). Oral pilocarpine for treatment of opioid-induced oral dryness in healthy adults. Journal of Dental Research, 83(5), 393–397. 10.1177/154405910408300508 [DOI] [PubMed] [Google Scholar]
- Grey A, Rix-Trott K, Horne A, Gamble G, Bolland M, & Reid IR (2011). Decreased bone density in men on methadone maintenance therapy: Bone density during methadone therapy. Addiction, 106(2), 349–354. 10.1111/j.1360-0443.2010.03159.x [DOI] [PubMed] [Google Scholar]
- Harris MTH, Lambert AM, Maschke AD, Bagley SM, Walley AY, & Gunn CM (2022). “No home to take methadone to”: Experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. Journal of Substance Abuse Treatment, 135, 108655. 10.1016/j.jsat.2021.108655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KA, Foot C, Levander XA, Cook R, Terashima JP, McIlveen JW, Korthuis PT, & McCarty D (2022). Treatment retention, return to use, and recovery support following COVID-19 relaxation of methadone take-home dosing in two rural opioid treatment programs: A mixed methods analysis. Journal of Substance Abuse Treatment, 141, 108801. 10.1016/j.jsat.2022.108801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y-I, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, & Ling W (2014). Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction (Abingdon, England), 109(1), 79–87. 10.1111/add.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huissoud T, Rousson V, & Dubois-Arber F (2012). Methadone treatments in a Swiss region, 2001-2008: A registry-based analysis. BMC Psychiatry, 12, 238. 10.1186/1471-244X-12-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Compton WM, Han B, Baldwin G, & Volkow ND (2022). Methadone-Involved Overdose Deaths in the US Before and After Federal Policy Changes Expanding Take-Home Methadone Doses From Opioid Treatment Programs. JAMA Psychiatry, 79(9), 932. 10.1001/jamapsychiatry.2022.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Alford DP, Malabanan A, Holick MF, & Samet JH (2006). Low bone density in patients receiving methadone maintenance treatment. Drug and Alcohol Dependence, 85(3), 258–262. 10.1016/j.drugalcdep.2006.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N, Maniates H, Hulsey E, Smith JS, DiDomenico E, Stuart EA, Saloner B, & Bandara S (2022). Shifting Medication Treatment Practices in the COVID-19 Pandemic: A Statewide Survey of Pennsylvania Opioid Treatment Programs. Journal of Addiction Medicine, 16(6), 645–652. 10.1097/ADM.0000000000000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N, Rivera BD, Jent V, Keyes KM, Jones CM, & Cerdá M (2022). Has the treatment gap for opioid use disorder narrowed in the U.S.?: A yearly assessment from 2010 to 2019”. International Journal of Drug Policy, 103786. 10.1016/j.drugpo.2022.103786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Bruneau J, & Stewart J (2003). Understanding polydrug use: Review of heroin and cocaine co-use. Addiction (Abingdon, England), 98(1), 7–22. 10.1046/j.1360-0443.2003.00236.x [DOI] [PubMed] [Google Scholar]
- Levander XA, Hoffman KA, McIlveen JW, McCarty D, Terashima JP, & Korthuis PT (2021). Rural opioid treatment program patient perspectives on take-home methadone policy changes during COVID-19: A qualitative thematic analysis. Addiction Science & Clinical Practice, 16(1), 72. 10.1186/s13722-021-00281-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levander XA, Pytell JD, Stoller KB, Korthuis PT, & Chander G (2022). COVID-19 related policy changes for methadone take-home dosing: A multistate survey of opioid treatment program leadership. Substance Abuse, 43(1), 633–639. 10.1080/08897077.2021.1986768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil R, Puri N, Boyd J, Mayer S, Hayashi K, & Small W (2020). Understanding concurrent stimulant use among people on methadone: A qualitative study. Drug and Alcohol Review, 39(3), 209–215. 10.1111/dar.13049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine 2019. (2019). Medications for Opioid Use Disorder Save Lives (Leshner AI & Mancher M, Eds.; p. 25310). National Academies Press. 10.17226/25310 [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. (2012). Principles of Drug Addiction Treatment: A Research-Based Guide: Third Edition: (686332012-001) [Data set]. American Psychological Association. 10.1037/e686332012-001 [DOI] [Google Scholar]
- O’Connor AM, Cousins G, Durand L, Barry J, & Boland F (2020). Retention of patients in opioid substitution treatment: A systematic review. PLOS ONE, 15(5), e0232086. 10.1371/journal.pone.0232086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SL, Copeland AL, Kopak AM, Hoffmann NG, Herschman PL, & Polukhina N (2015). Predictors of patient retention in methadone maintenance treatment. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors, 29(4), 906–917. 10.1037/adb0000090 [DOI] [PubMed] [Google Scholar]
- Ramli FF, Syed Hashim SA, & Mohd Effendy N (2021). Factors Associated with Low Bone Density in Opioid Substitution Therapy Patients: A Systematic Review. International Journal of Medical Sciences, 18(2), 575–581. 10.7150/ijms.52201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Kosich O, Andraka-Christou B, Totaram R, Alamo J, & Nadig M (2020). Comparing Reasons for Starting and Stopping Methadone, Buprenorphine, and Naltrexone Treatment Among a Sample of White Individuals With Opioid Use Disorder. Journal of Addiction Medicine, 14(4), e44–e52. 10.1097/ADM.0000000000000584 [DOI] [PubMed] [Google Scholar]
- Reisinger HS, Schwartz RP, Mitchell SG, Peterson JA, Kelly SM, O’Grady KE, Marrari EA, Brown BS, & Agar MH (2009). Premature Discharge from Methadone Treatment: Patient Perspectives. Journal of Psychoactive Drugs, 41(3), 285–296. 10.1080/02791072.2009.10400539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, Banys P, & Hall SM (2000). Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: A randomized controlled trial. JAMA, 283(10), 1303–1310. 10.1001/jama.283.10.1303 [DOI] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, & Pastor-Barriuso R (2017). Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ, 357. 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2021a). Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. HHS Publication No. PEP21-07-01-003, NSDUH Series H-56. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, 156. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2021b). Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63. Publication No. PEP21-02-01-002. Rickville, MD: Substance Abuse and Mental Health Services Administration, 332. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2022, December 16). Medications for the Treatment of Opioid Use Disorder. Federal Register. https://www.federalregister.gov/documents/2022/12/16/2022-27193/medications-for-the-treatment-of-opioid-use-disorder
- Suen LW, Castellanos S, Joshi N, Satterwhite S, & Knight KR (2022). “The idea is to help people achieve greater success and liberty”: A qualitative study of expanded methadone take-home access in opioid use disorder treatment. Substance Abuse, 43(1), 1147–1154. 10.1080/08897077.2022.2060438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, & Garrison-Diehn C (2016). Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of Addictive Diseases, 35(1), 22–35. 10.1080/10550887.2016.1100960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titsas A, & Ferguson MM (2002). Impact of opioid use on dentistry. Australian Dental Journal, 47(2), 94–98. 10.1111/j.1834-7819.2002.tb00311.x [DOI] [PubMed] [Google Scholar]
- Tong A, Sainsbury P, & Craig J (2007). Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. International Journal for Quality in Health Care, 19(6), 349–357. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- Williams AR, Nunes EV, Bisaga A, Levin FR, & Olfson M (2019). Development of a Cascade of Care for responding to the opioid epidemic. The American Journal of Drug and Alcohol Abuse, 45(1), 1–10. 10.1080/00952990.2018.1546862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador D, Lyons Wall PM, & Webster I (1996). High sugar intake in a group of women on methadone maintenance in south western Sydney, Australia. Addiction (Abingdon, England), 91(7), 1053–1061. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


