Figure 10.
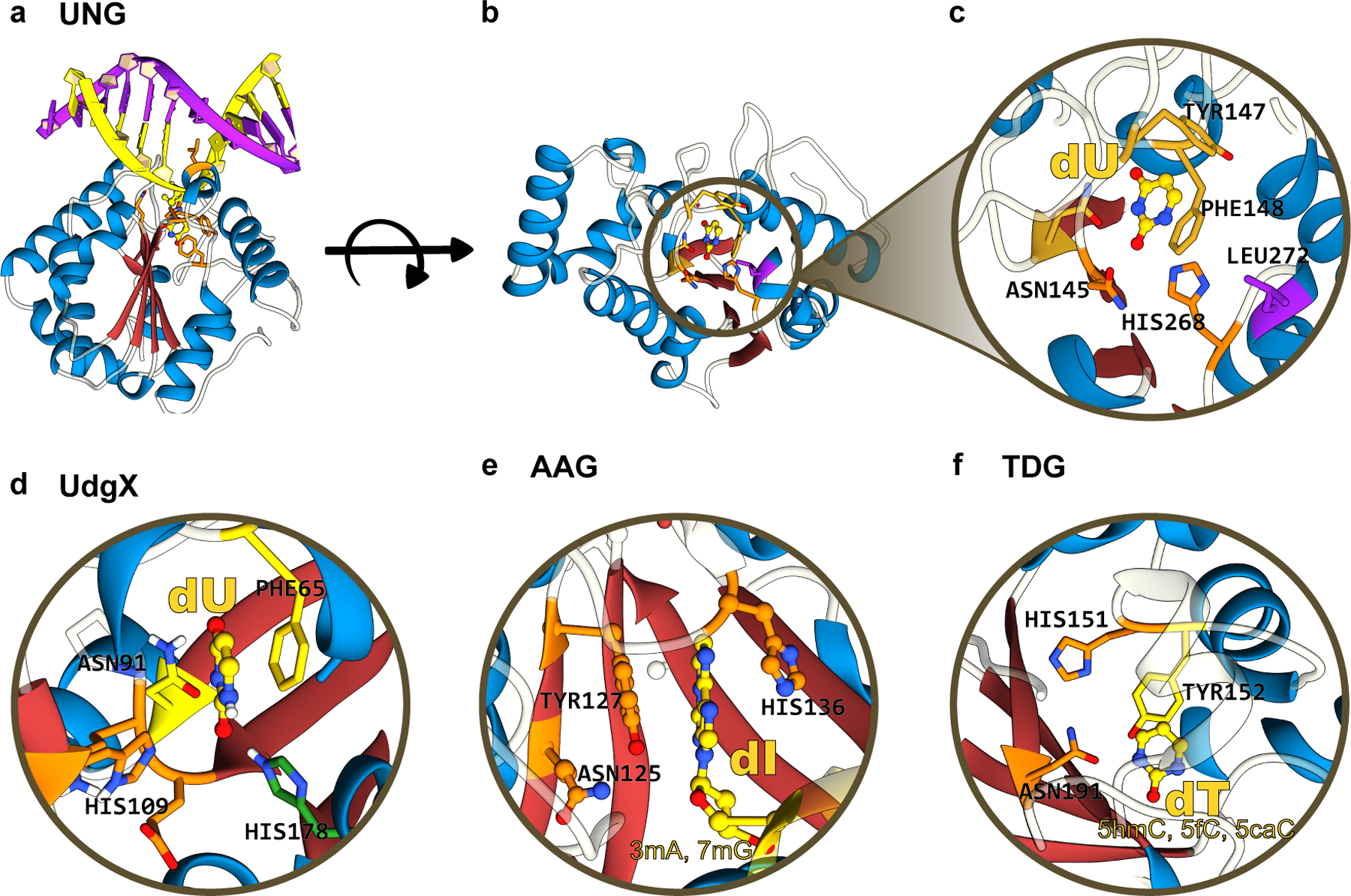
(a) Side-view, (b) top-view, and (c) zoomed-in structures of human UNG, which form the basis of current “transversion” base editors. The α/β fold of these glycosylases is color-coded as follows: the β-sheet core is in red and peripheral α-helices are in blue. (d), (e), and (f) correspond to the zoomed-in structure of UdgX, AAG, and TDG, respectively, highlighting the conserved β-stranded core. The aromatic residues(Phe/Tyr) responsible for stabilizing the “everted” nucleotide as well as the His and Asn residues critical for the excision reaction are annotated. UDG, UdgX, AAG, and TDG structures correspond to PDB ID: 1SSP(124), 6AIL(125), 1EWN(126), and 3UOB(177) respectively. (cf. Figure 9)
