Figure 7.
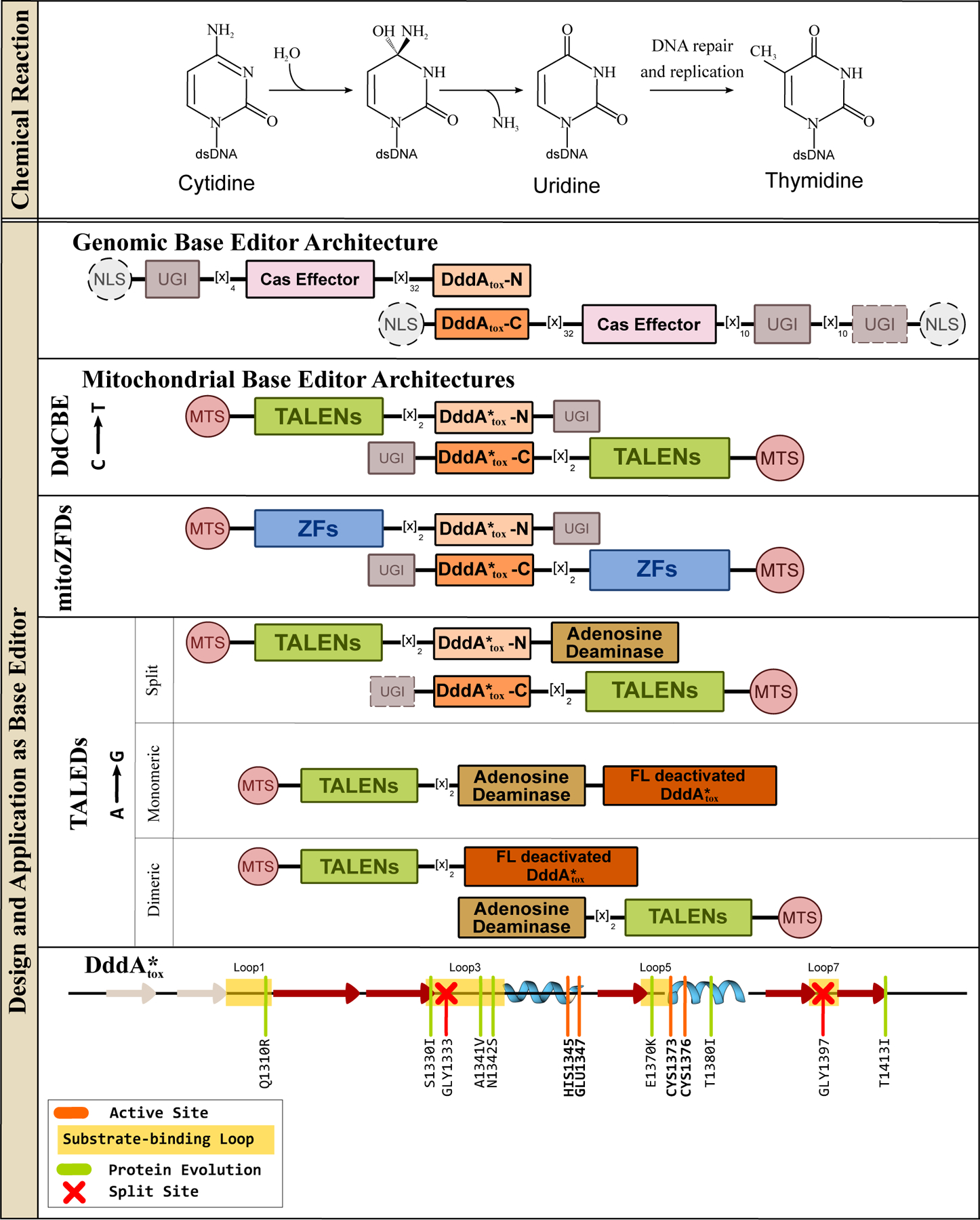
Deaminase toxin A (DddAtox), a dsDNA cytidine deaminase, and its design and application in genomic and mitochondrial base editing. (Top) DddAtox can hydrolytically deaminate cytosines in dsDNA to yield uridine, when is then processed into thymidine via DNA replication and/or repair processes. Overall, this reaction gives rise to a C·G→T·A base pair conversion. (Middle) Representative DdCBE, mitoZFDs, and DddAtox-based ABEs (TALEDs) architectures are shown, with essential and non-essential components indicated with solid and dashed outlines, respectively. (Bottom) Secondary structure alignment of the DddAtox enzyme is shown, with an emphasis on the core CDA fold. Locations of the substrate-binding loops and active site residues are indicated, and key mutations discovered using directed evolution to enhance certain properties of the corresponding DdCBE are shown in light green and the split sites are indicated with red crosses.
