Figure 8.
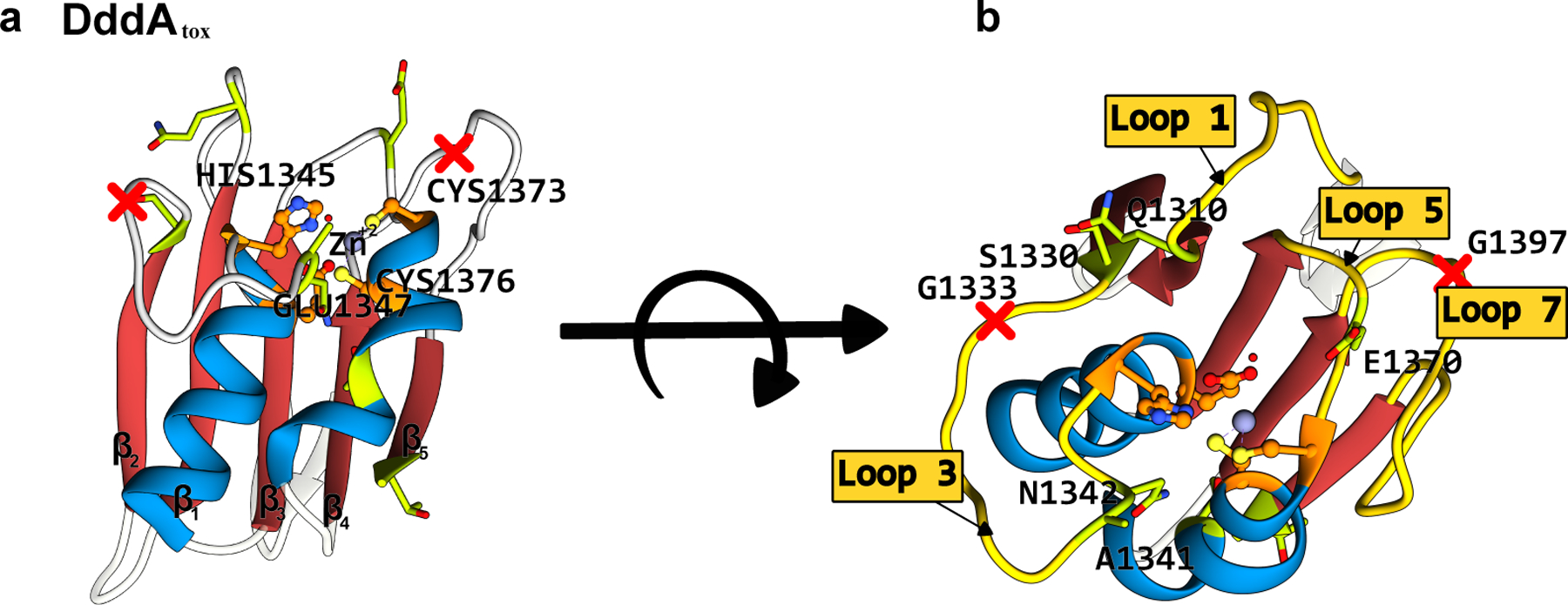
Structure of the B. cenocepacia DddAtox enzyme (PDB ID: 6U08)(102). (a) Side-view and (b) top-view. The conserved CDA fold is color-coded as follows: the β-sheet core is red and the peripheral α-helices are blue. The active site residues are shown in orange, with the mutations that have enhanced certain properties of the deaminase (when used as a base editor) shown in green. The split sites that were used to divide the enzyme into two inactive, non-toxic halves are shown as red crosses. (cf. Figure 7)
