Figure 6. Tiam1 regulates neuropathic pain behaviors via Rac1 signaling.
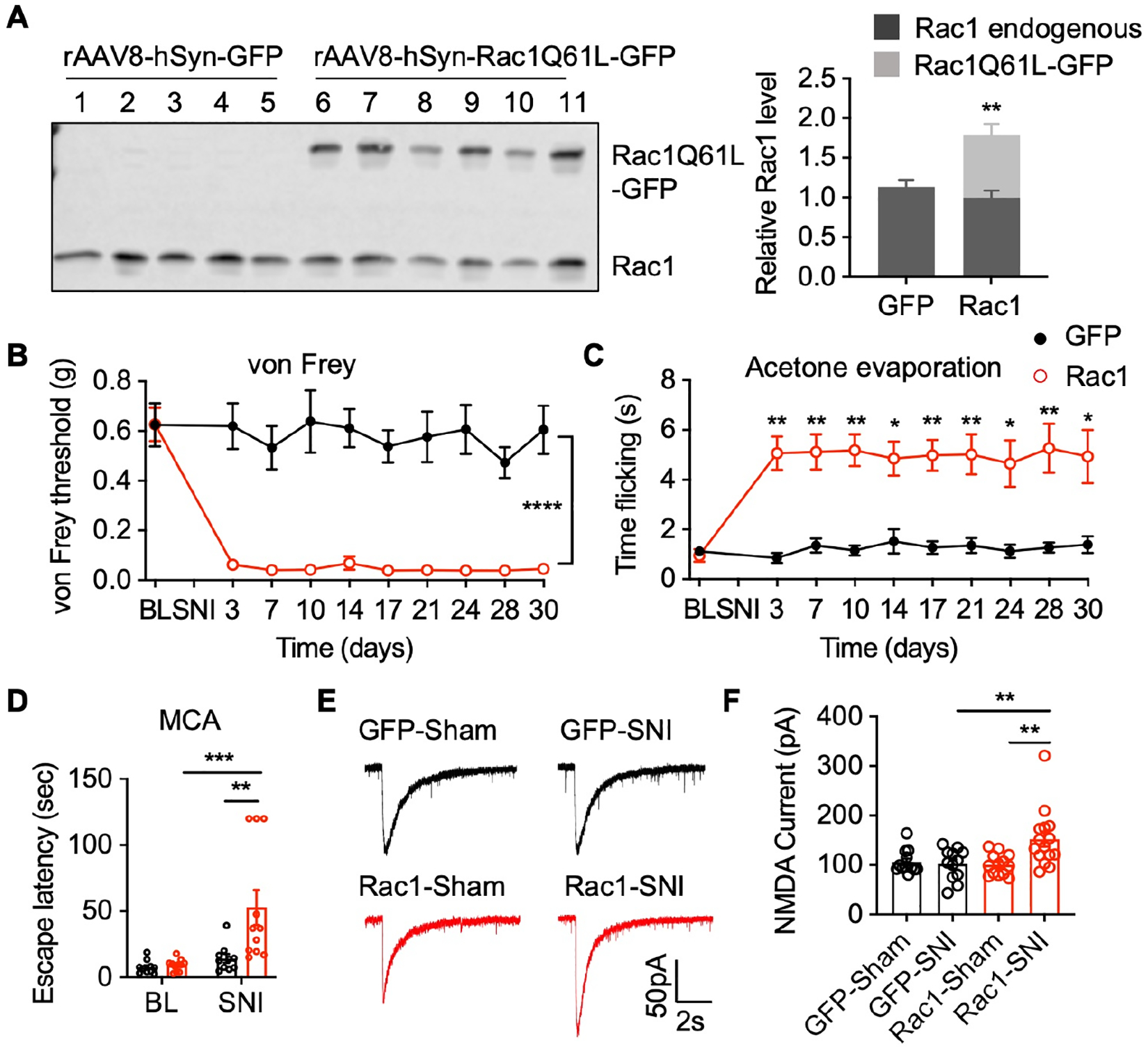
(A) Western blots and quantification of endogenous Rac1 and constitutively-active Rac1Q61L-GFP transgene expression in the spinal dorsal horn of global Tiam1 KO mice infected with control rAAV8-hSyn-GFP (GFP) or rAAV8-hSyn-Rac1Q61L-GFP (Rac1) via intra-spinal dorsal horn microinjection. Student t-test (n = 5–6). Endogenous Rac1 was used as the loading control. (B-D) Rac1Q61L expression in the spinal dorsal horn reverses the diminished neuropathic pain phenotype in global Tiam1 KO mice. von Frey withdrawal thresholds (B), time flicking with acetone evaporation (C), and escape latency in the MCA assay (D) of global Tiam1 KO mice infected with rAAV8-hSyn-GFP (GFP) or rAAV8-hSyn-Rac1Q61L-GFP (Rac1) before and after SNI. Two-way ANOVA analysis followed by Dunnett’s post-hoc test (n = 5–11 mice/group. von Frey: P < 0.0001, F9,130 = 6.812; acetone: P = 0.0308, F9,124 = 2.141; MCA: P = 0.0013, F1,38 = 12.07).
(E and F) Rac1Q61L expression in the spinal dorsal horn rescues the diminished NMDAR activity in global Tiam1 KO mice. Original current traces and mean changes in NMDAR currents elicited by puff application of 100 mM NMDA to spinal dorsal horn neurons in global Tiam1 KO mice infected rAAV8-hSyn-GFP (GFP) or rAAV8-hSyn-Rac1Q61L-GFP (Rac1) 3 weeks after SNI surgery. One-way ANOVA followed by Tukey’s post-hoc test (n = 12–15 neurons. P = 0.0015, F3,48 = 5.960).
Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
