Figure 1.
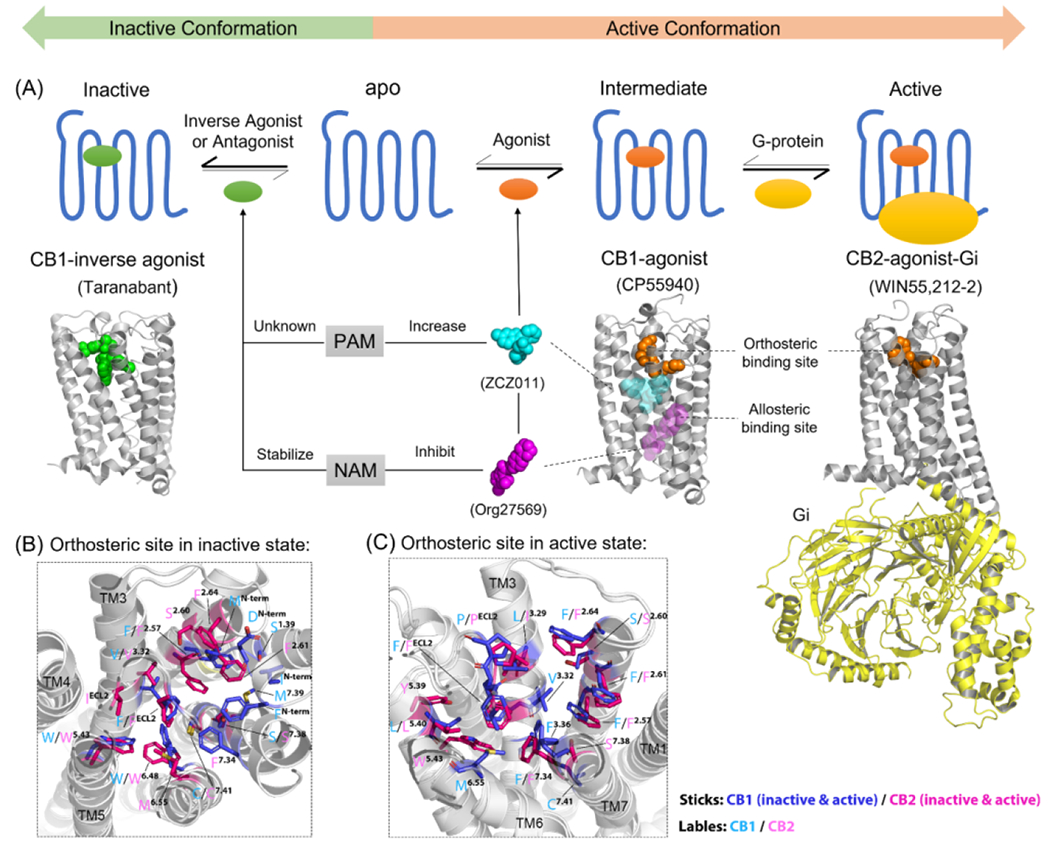
Active and inactive states of cannabinoid receptors (CBRs). (a) Brief overview of how orthosteric ligands and allosteric modulators (AMs) can alter the active/inactive states of CBRs in general. All the 3D structure of CBRs are shown in gray. The X-ray crystal structure of a CB1-inverse agonist (Taranabant, green sphere) complex [Protein Data Bank (PDB): 5U09] is used to represent the inactive conformation of CBR. The X-ray crystal structure of a CB1-agonist (CP55940, orange sphere) complex (PDB: 6kqi) is used to represent the intermediate state. The cryo-electron microscopy (cryo-EM) structure of a CB2-agonist (WIN55,212–2, orange sphere)-Gi protein (yellow cartoon) complex (PDB: 6pt0) is used to represent the active state. PAM (ZCZ011, cyan sphere) and NAM (Org27569, magenta sphere) were used as examples to show the allosteric binding sites on CBRs. However, there are various positions of the binding sites of positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs); in addition, there is not enough evidence to identify whether PAM and NAM could bind to the same sites. The orthosteric binding sites of CBRs in (b) inactive and (c) active states. The 3D structures of CBRs are shown as white cartoons. The important ligand-interacting residues within 4 Å of the orthosteric ligands in the orthosteric binding site are shown in blue (inactive/active CB1R, PDB: 5U09/7wv9), and magenta (inactive/active CB2R, PDB: 5zty/6pt0) with cyan (CB1) and pink (CB2) labels. Figure based on information from 15.
