Figure 2.
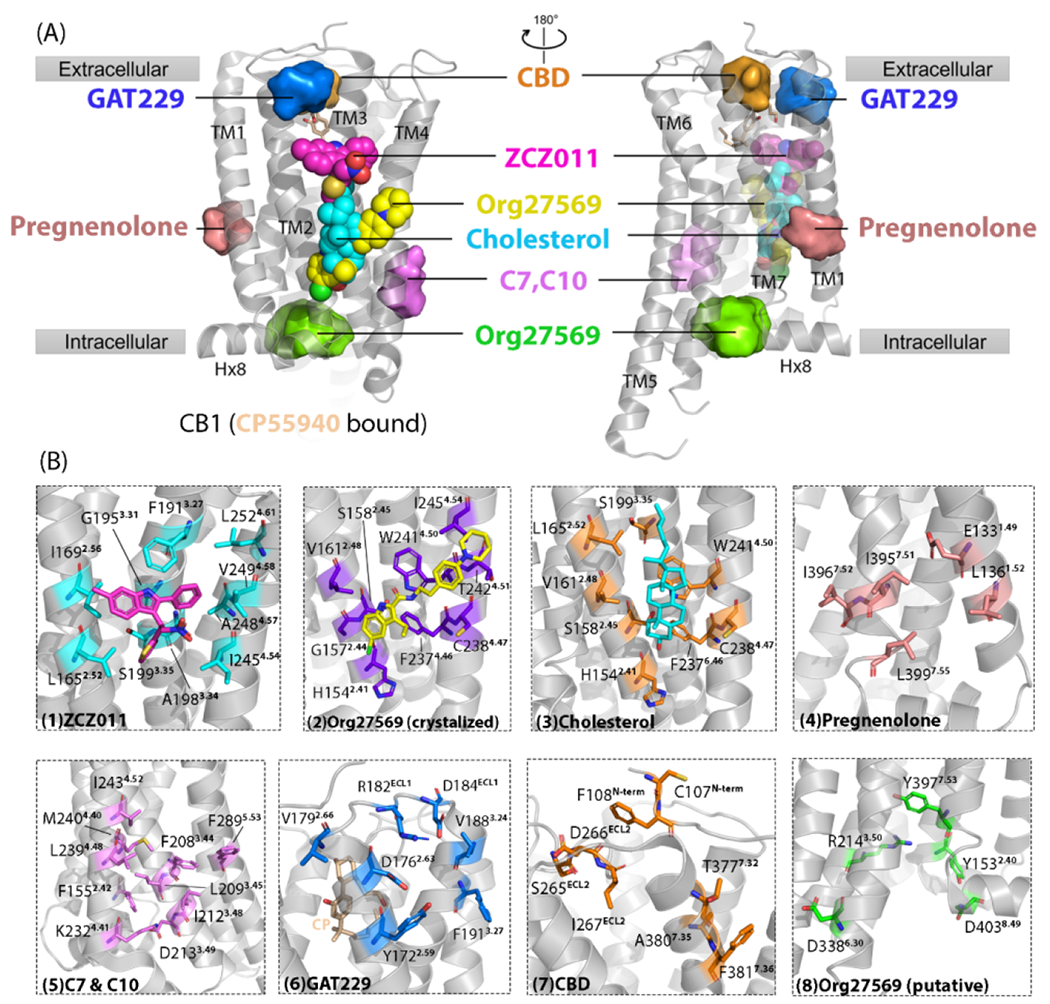
Cannabinoid receptor 1 (CB1) reported/putative allosteric binding sites. (a) Cryo-electron microscopy (cryo-EM) structure of CB1 (CP55940 bound) is shown in gray [Protein Data Bank (PDB): 7wv9]. CP55940 is represented as yellow sticks. Seven allosteric sites are shown. Three allosteric sites reported by high-resolution X-ray crystal or cryo-EM structures are shown as spheres: yellow sphere, Org27569 binding site; cyan sphere, cholesterol binding site; magenta sphere, ZCZ011 binding site. Other allosteric sites shown as surfaces are putative ones revealed via computational approaches, such as molecular dynamics simulations and molecular docking, and validated by site-directed mutagenesis. (b) Magnified details for each site shown in (a). From left to right, the detailed ligand-interacting residues in each allosteric site are: (1) ZCZ011 (cyan sticks) in cryo-EM structures (PDB: 7wv9), (2) co-crystalized Org27569 (purple sticks) (PDB: 6kqi), (3) co-crystallized cholesterol (orange sticks) (PDB: 5xra), (4) pregnenolone (salmon sticks), (5) c7 and C10 (violet sticks), (6) GAT229 (blue sticks), (7) CBD (orange sticks), and (8) Org27569 putative site (green sticks).
