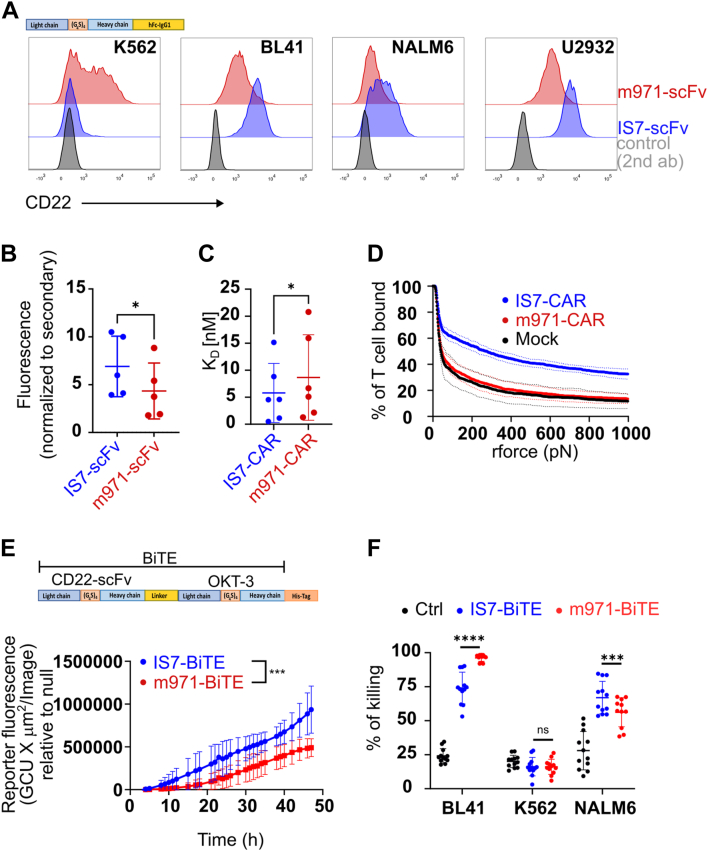
Figure 2.
Comparison of m971- and IS7-scFv using different formats.A, an erythroid/myelogenous leukemic cell line (K562), two B-cell lymphoma cell lines (BL41 and U2932), one B-ALL cell line (NALM6). B, B-ALL patient samples were labeled with IS7 and m971 soluble scFvs, then incubated with an anti-Fc secondary antibody. Geometric MFI (gMFI) was normalized to the Fc-only controls. (mean ± SD, paired two-tailed t test, n = 5. ∗p < 0.05). C, dissociation assay; IS7 and m971-CAR T cells were labeled with a dilution series of CD22-Fc chimeras. Secondary labeling with anti-Fc was performed at a constant concentration. (median ± interquartile range, Wilcoxon matched-pairs signed rank test, n = 6, ∗p < 0.05). D, in the z-Movi assay, CAR-transduced T cells were incubated with bound target (BL41) cells and subjected to increasing pressure until dissociation (two replicates from a single donor). E, Jurkat E6-NFAT-GFP reporter cells were cocultured with BL41 target cells (E:T = 1:1), in the presence of equal amounts of IS7- or m971-BiTEs (based upon His-tag labeling intensity on the Western blots, Fig. S1C). These were cultured in an IncuCyte apparatus, and the resulting reporter signal (GFP) was recorded over time. The signal was normalized against null controls (i.e., HEK supernatants without BiTEs). F, IS7- and m971-BiTE were cocultured with activated T cells and the indicated CD22+ targets for 8 h at an E:T ratio = 1:1. BiTE-containing supernatant was standardized to 50% of the highest concentration recorded (Fig. S1D), then diluted 10-fold. (paired t test, mean ± SD, n = 4 donors in triplicate). CAR, chimeric antigen receptor; B-ALL,B-cell acute lymphoblastic leukemia; BiTE,bispecific T-cell engager; scFv, single chain variable fragment.