Abstract
Objective: To retrospectively analyze the iodine nutritional status in patients with nodular goiter (NG) and investigate a possible association between urinary iodine levels and thyroid function indices. Methods: A total of 173 patients diagnosed with nodular goiter in the Fourth Hospital of Hebei Medical University from January 2019 to May 2021 were selected as the NG group, and 172 healthy individuals without thyroid diseases were selected after a physical examination as a control group. The data of all the participants were retrospectively assessed to explore the association between urinary iodine levels and thyroid function indices. The content of urinary iodine in the two groups was compared, and the correlation of urinary iodine levels with thyroid stimulating hormone (TSH), free triiodothyronine (FT3), and free thyroxine (FT4) in the NG group was evaluated. Results: The level of urinary iodine in the NG group was 163.97 ± 113.75 μg/L, which was higher than 121.47 ± 53.75 μg/L in the control group (P < 0.05). The iodine excess rate in females was higher than that in males (P < 0.05). The results of Pearson correlation analysis showed that the amount of urinary iodine in patients with hyperthyroidism with different urinary iodine statuses was negatively correlated with the level of TSH and positively correlated with levels of FT3 and FT4. Conclusion: There is a significant association between urinary iodine levels and thyroid hormone levels in NG patients. Therefore, regular monitoring of urinary iodine levels is essential for the appropriate use of iodine supplementation.
Keywords: Urinary iodine, nodular goiter, thyroid function, thyroid disease
Introduction
Goiter is a global health problem caused by abnormal iodine intake, with an increasing incidence, affecting nearly 10% of the global population. It is mainly characterized by diffuse or nodular enlargement of the thyroid gland [1]. Nodular goiter (NG) is the excessive growth of thyroid tissue caused by structural and functional changes in multiple regions of the thyroid [2]. Evidence indicates that abnormal intake of essential trace elements (iodine and selenium) is one of the main risk factors for NG [3].
Iodine is an indispensable nutrient for the human body [4]. Iodine deficiency, along with vitamin A deficiency and iron deficiency, is one of the world’s three major micronutrient deficiency diseases [5]. Iodine is an essential element necessary for the synthesis of thyroxine. The endocrine system utilizes iodine for the synthesis of thyroid hormones [6]. Both insufficient and excessive iodine intake can have an impact on the synthesis of thyroid hormones, leading to various thyroid dysfunctions and diseases. Alterations in thyroid hormone levels in the body can lead to thyroid dysfunction, autoimmune thyroid abnormalities, goiter, benign and malignant thyroid nodules, and can even affect other tissues and organs [7]. In the past, insufficient iodine intake was typical in China. Since iodized salt was popularized in 1995, the situation of iodine deficiency has significantly improved. However, in recent years, the incidence of goiter in China has shown an annual increase, and the iodine levels in adolescents in some areas have exceeded recommended limits [8,9]. A recent study highlighted that excessive iodine intake may also cause thyroid diseases [10]. For example, the use of high-dose iodine for preventive treatment in areas with insufficient iodine may sometimes lead to iodine excess and an increased risk of papillary thyroid cancer. Similarly, areas with high iodine content may have a high incidence of papillary thyroid carcinoma. Urinary iodine level is an important index of the iodine nutritional status of the body [11]. A recent study has highlighted that iodine deficiency can result in abnormal synthesis of thyroid hormones, leading to thyroid diseases, especially hyperthyroidism [12]. Therefore, the iodine content is related to thyroid function in patients with NG. However, our understanding of the relationship between thyroid function indices and urine iodine levels in NG requires improvement. There are few such studies investigating this relationship, and the conclusions are not yet unified. Based on this, this study aims to explore the relationship between urinary iodine levels and thyroid function in patients with NG.
In the present study, the level of urinary iodine was measured to evaluate the iodine nutrition status of the local population. This retrospective study investigated the correlation between urinary iodine levels and thyroid function in both NG patients and healthy individuals. Our findings may provide a scientific basis for prevention and treatment of the harm caused by excessive iodine.
Methods
Participants
In this retrospective study, 173 patients with NG treated in the Fourth Hospital of Hebei Medical University from January 2019 to May 2021 were selected as an NG group. 172 healthy individuals who underwent physical examination that ruled out thyroid diseases in the same hospital during the same period were selected as a control group. This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University. The diagnostic criteria for NG were based on thyroid color Doppler ultrasonography, which showed diffuse thyroid lesions that could be clearly distinguished from the surrounding thyroid tissue by imaging and exhibited single or multiple nodules.
Inclusion and exclusion criteria
Inclusion criteria: 1) local permanent residents who were 18~70 years old; 2) healthy individuals without thyroid diseases were taken as the control group, and patients diagnosed with NG were included in the NG group. The diagnosis of NG was based on the 2020 Chinese thyroid nodule ultrasound risk stratification guidelines: C-TIRADS [13]. Exclusion criteria: 1) patients with hepatorenal insufficiency caused by pregnancy, diabetes, or other diseases; 2) patients with iodine contrast media history; 3) patients with medical records that disallowed iodine or controlled the iodine intake.
Assessment of urinary iodine
Spot urine samples were collected from all participants in the morning (fasting time > 8 h) using polyethylene plastic tubes. Participants were instructed to maintain their regular rest and diet before sample collection and to avoid consuming iodine-containing food, iodine supplements and medications. Urinary iodine was assessed by the urinary iodine detector SR-I-100 (Shenrui Biological).
Determination of thyroid function indices
Peripheral venous blood samples were collected from all participants in the morning (fasting time > 8 h). Then, the blood samples were centrifuged for 10 min at 3000 rpm. Serum levels of thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), and free thyroxine (FT4) were measured using the chemiluminescence method. The Siemens XP Centaur chemiluminescence instrument was used for the measurements.
Evaluation standard of urinary iodine status
The iodine nutrition status was evaluated according to the standards formulated by the World Health Organization, the United Nations Children’s Fund, and the International Council for the Control of IDD [14]. Specifically, urinary iodine < 100 μg/L is considered as iodine deficiency in children and adults, 100-199 μg/L as adequate iodine, 200-299 μg/L as more than adequate, and > 300 μg/L as iodine excess.
Statistical analysis
SPSS 24.0 (SPSS Inc., U.S.A.) was used for statistical analysis. Pearson’s correlation coefficient was employed to assess the associations between iodine secretion and serum TSH, TH3, and TH4. The measured data were subject to a normal distribution, and were expressed as mean ± SEM. The Chi-square test was employed to examine the differences between categorical variables. Wilcoxon test or paired t-test was employed for comparison between groups. Kendall’s tau analysis was applied to explore the associations between urinary iodine and TSH, TH3, and TH4. P value < 0.05 was considered significant.
Results
Characteristics of the study population
The NG group exhibited a lower body mass index and a higher proportion of participants with drinking problems compared to the control group (P < 0.05). Moreover, there was no significant difference in sex, age, or seafood eating between the two groups (P < 0.05) (Table 1).
Table 1.
Descriptive features of the study population
| Characteristic | Controls (n=172) | NG (n=173) | t/χ2 | P |
|---|---|---|---|---|
| Sex, n (%) | - | |||
| Male | 71 (41.28) | 69 (39.88) | ||
| Female | 101 (58.72) | 104 (60.12) | ||
| Age (years), n (%) | - | |||
| ≤ 40 | 85 (49.42) | 86 (49.71) | ||
| 41-60 | 61 (35.47) | 50 (28.90) | ||
| ≥ 61 | 26 (15.11) | 37 (21.39) | ||
| BMI (kg/m2, Mean ± SD) | 28.39 ± 3.95 | 27.23 ± 2.78 | 0.240 | 0.006 |
| Seafood, n (%) | 0.908 | 0.386 | ||
| More than once a week | 24 (13.96) | 21 (12.21) | ||
| Once or twice a week | 132 (76.74) | 133 (77.33) | ||
| More than twice a week | 16 (9.30) | 18 (10.47) | ||
| Smoking, n (%) | 26 (15.17) | 35 (20.23) | 0.855 | 0.184 |
| Drinking, n (%) | 67 (38.95) | 98 (56.65) | 0.942 | 0.007 |
BMI: Body mass index; NG: nodular goiter. P-values were obtained using χ2 tests for categorical variables. Wilcoxon test was used for continuous variables.
Urinary iodine content in the two groups
There was no significant difference in the average age between the NG group and the control group (P > 0.05). The content of urinary iodine in the NG group was significantly higher than that in the control group (P < 0.05), as shown in Table 2 and Figure 1.
Table 2.
Comparison of UI content between the two groups
| Characteristic | Controls (n=172) | NG (n=173) | t | P |
|---|---|---|---|---|
| Average age | 44.37 ± 10.98 | 45.62 ± 11.31 | 1.033 | 0.115 |
| UI content (μg/L) | 121.47 ± 53.75 | 163.97 ± 113.75 | 0.906 | 0.001 |
UI: Urinary iodine; NG: nodular goiter. Wilcoxon test was used for continuous variables.
Figure 1.
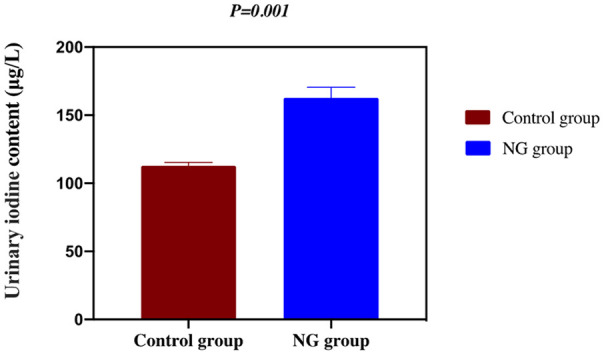
Urinary iodine content in NG and control groups. P < 0.01, control vs. NG. NG: nodular goiter.
Urinary iodine content in males and females
As shown in Table 3, the NG group showed no significant difference in the urinary iodine content or iodine deficiency rate between males and females (both P > 0.05), but the iodine excess rate in females was significantly higher than that in males (P < 0.05). In contrast, the control group exhibited no significant difference between males and females for all three indicators above (P > 0.05).
Table 3.
Urinary iodine content of males and females in the two groups [n (%), (x ± s)]
| Group | Female | Male | t/χ2 | P | |
|---|---|---|---|---|---|
| NG group (n=173) | UI content (μg/L) | 163.44 ± 67.89 | 169.50 ± 72.45 | 0.695 | 0.778 |
| Iodine deficiency rate | 46 | 9 | 0.880 | 0.645 | |
| Iodine excess rate | 57 | 11 | 0.641 | 0.004 | |
| Control group (n=172) | UI content (μg/L) | 122.13 ± 67.56 | 124.55 ± 65.40 | 0.252 | 0.712 |
| Iodine deficiency rate | 12 | 5 | 1.081 | 0.746 | |
| Iodine excess rate | 38 | 15 | 0.800 | 0.193 |
UI: Urinary iodine; NG: nodular goiter. P-values were obtained using χ2 tests for categorical variables. Wilcoxon test was used for continuous variables.
Correlation of urinary iodine content with FT3, FT4, and TSH in patients with NG
As shown in Figure 2, the Spearman correlation analysis showed that the content of urinary iodine in the NG group was positively correlated with FT3 (r=0.548, P=0.035) and FT4 (r=0.510, P=0.007), whereas it was negatively correlated with TSH (r=-0.631, P=0.027).
Figure 2.
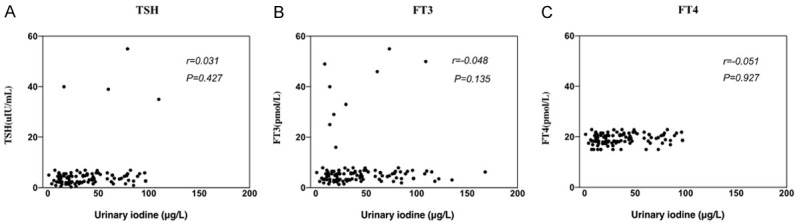
Relationship between urinary iodine content and thyroid function in the nodular goiter group. TSH: thyroid stimulating hormone; FT3: free triiodothyronine; FT4: free thyroxine.
Correlation of urinary iodine excretion with thyroid function
Fifteen NG participants, age 34 ± 55 years (median age 43 years) were selected for further investigation. The urine samples of the 15 participants were collected to assess urinary iodine excretion by measuring the levels of urinary iodine and creatinine in urine sections. Pearson’s correlation analysis was performed to explore the association between urinary iodine excretion and thyroid function indices. Figure 3 shows the correlation coefficients between urinary iodine excretion and thyroid function, as assessed by urinary TSH, FT3, and FT4 in the study participants. A negative correlation was detected in 10 participants, while a positive relationship was found in 5 participants.
Figure 3.
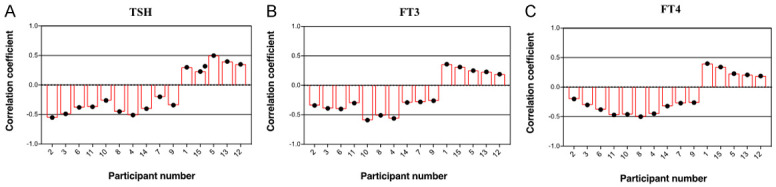
Correlation coefficients between urinary iodine excretion and thyroid function in 15 patients with nodular goiter. Correlations were analyzed using Pearson’s correlation coefficients. TSH: thyroid stimulating hormone; FT3: free triiodothyronine; FT4: free thyroxine.
Average correlation coefficients between urinary iodine excretion and TSH
The correlation between urinary iodine excretion and TSH showed a significant shift from negative to positive among the participants (P=0.002) when they were assessed based on average annual iodine excretion (Figure 4).
Figure 4.
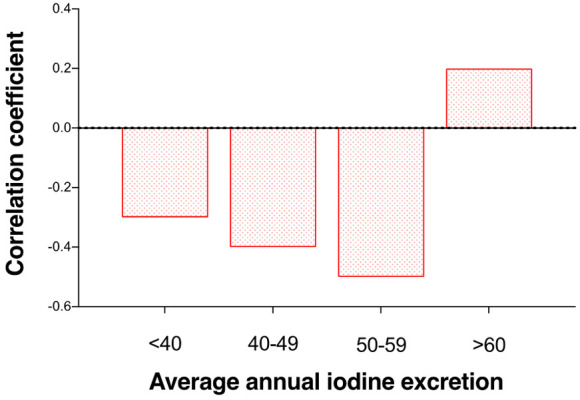
Average correlation coefficients (x ± s) between urinary iodine excretion and thyroid stimulating hormone in 15 patients with nodular goiter. Participants were categorized according to average annual urinary iodine excretion.
Discussion
In recent years, there has been an increase in the incidence of nodular goiter (NG). The prevalence of NG in the elderly has reached 7%, with women comprising the majority of cases [15,16]. In terms of pathophysiology, the early stage of NG is usually characterized by simple goiter [17]. After further development, thyroid follicles undergo repeated proliferation, degeneration and atrophy, leading to the formation of nodules and fibrous septa. There was no obvious manifestation in the early stage of NG, but in the later stage, the goiter nodule is enlarged and often gives oppressive symptoms [18]. As NG is often asymptomatic or has nonspecific symptoms at an early stage, most of the cases in this study were diagnosed due to the presence of neck lumps and compressive symptoms. Therefore, early diagnosis of NG is crucial for timely treatment and management of the disease.
The pathogenesis of NG has yet to be fully clarified, and it may be related to the environment, age, hormones, iodine nutritional status, or other factors [19]. In this study, the average content of urinary iodine in the control group was 121.47 ± 53.75 μg/L, ranging between 100 ug/L and 199 ug/L according to the evaluation standard of urinary iodine, so we believe that the average iodine nutrition status of the residents in this area is in the normal range. According to the evaluation standard of iodine nutrition status recommended by the World Health Organization [20], the urinary iodine content of the NG population in this area (163.97 ± 113.75 μg/L) was in the normal range. This might be representative of the iodine nutrition status of the entire population in this area. As a trace element closely related to geography, it is of great clinical significance to establish the reference range of urinary iodine in this area [21]. Compared to healthy individuals in this area, the content of urinary iodine in the NG population was significantly higher, and the reported iodine intake was basically consistent with the “U” curve of thyroid disease [22]. When the body is in a state of iodine deficiency, the thyroid gland enhances the use of iodine due to negative feedback, which leads to the production of TSH. It then repeatedly stimulates the thyroid gland, resulting in compensatory hyperplasia and enlargement [23,24]. When iodine deficiency persists in the body, the synthesis of T3 increases while that of T4 decreases due to the body’s low demand for iodine as T3 and the high demand for iodine as T4. When the concentration of T4 decreases, further negative feedback can lead to the increase of TSH secretion, which aggravates thyroid hyperplasia, resulting in NG [23,24].
Further research has found that high iodine can also increase the risk of NG [25]. It has been suggested that increased iodine uptake can inhibit thyroid peroxidase activity, leading to a decrease in the coupling and iodination processes, and ultimately resulting in a decrease in the synthesis of T3 and T4. This decrease in hormone synthesis can further trigger a negative feedback loop that leads to an increase in TSH secretion and the development of goiter [26]. In addition, sodium-iodide symporter (NIS) and thyrotropin receptor are expressed in the basolateral membrane of thyroid epithelium [27]. NIS is responsible for iodine uptake and plays a crucial role in iodine storage within the thyroid follicles. Research has shown that the location of NIS in the thyroid epithelium of patients with NG is abnormal [28]. Incorrect cytoplasmic localization of NIS can induce NG even under the condition of normal iodine or high iodine, resulting in low iodine in the follicle cavity [29].
In this study, we also found that in NG patients, the iodine overdose rate in women was significantly higher than that of men, but this was not true in healthy controls. Emerging evidence indicates that the incidence of NG is related to sex [21], which is consistent with the results of this study, but the specific mechanism remains to be further studied. In this study, the increase in the iodine excess rate in females with NG may be related to local dietary patterns, but further prospective studies are needed for clarification.
At present, there are many studies on iodine deficiency and thyroid diseases. Iodine deficiency may lead to a lack of thyroxine synthesis [30,31]. In recent years, studies have highlighted the negative effects of simple iodine supplementation. Other groups have reported that excessive iodine intake can lead to abnormal thyroid hormone levels and thyroid dysfunction [32]. In addition, both acute and chronic excessive iodine intake can have adverse effects on embryonic growth and development, human intelligence, and thyroid function [33,34]. Therefore, close monitoring of urinary iodine levels is necessary during iodine supplementation to ensure a reasonable and scientific-based approach.
However, this study has some limitations. The sample size in this study is small, and the thyroid function of the NG group was not analyzed by subgroups. In future studies, the sample size can be expanded, and subgroup analysis can be carried out to further clarify the correlation between urinary iodine and thyroid function.
To sum up, the population in the studied area appeared to have in a normal level of iodine, but NG patients in this area had higher levels of urinary iodine compared to healthy individuals. The content of urinary iodine is closely related to thyroid function. Regular monitoring of urinary iodine levels in patients with hyperactivity has significant clinical importance as it can guide the scientific supplementation of iodine.
Acknowledgements
The Project of Medical Science Research in Hebei Province (No. 20170752).
Disclosure of conflict of interest
None.
References
- 1.de Figueiredo WLD, Lopes EF, Jezini DL, Marçal LN, de Assunção EN, Ribeiro Rodrigues PR, José da Mota A, Carvalho DM, Filho SA, Lopes Botelho JB. Differential gene expression profile of multinodular goiter. PLoS One. 2022;17:e0268354. doi: 10.1371/journal.pone.0268354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toni R, Barbaro F, Di Conza G, Trimarchi F. Nodular goiter and laryngeal anatomic variant in two portraits of the hellenistic dramatist Menander. J Endocrinol Invest. 2022;45:2203–2204. doi: 10.1007/s40618-022-01772-z. [DOI] [PubMed] [Google Scholar]
- 3.Yuan L, Zhao P, Lin X, Yu T, Diao R, Ning G. T1 mapping and reduced field-of-view DWI at 3.0 T MRI for differentiation of thyroid papillary carcinoma from nodular goiter. Clin Physiol Funct Imaging. 2023;43:137–145. doi: 10.1111/cpf.12803. [DOI] [PubMed] [Google Scholar]
- 4.Andersson M, Braegger CP. The role of iodine for thyroid function in lactating women and infants. Endocr Rev. 2022;43:469–506. doi: 10.1210/endrev/bnab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbert G, England C, Perry R, Whitmarsh A, Moore T, Searle A, Chotaliya S, Ness A, Beasley M, Atkinson C. Impact of low iodine diets on ablation success in differentiated thyroid cancer: a mixed-methods systematic review and meta-analysis. Clin Endocrinol (Oxf) 2022;97:702–729. doi: 10.1111/cen.14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaker L, Razvi S, Bensenor IM, Azizi F, Pearce EN, Peeters RP. Hypothyroidism. Nat Rev Dis Primers. 2022;8:30. doi: 10.1038/s41572-022-00357-7. [DOI] [PubMed] [Google Scholar]
- 7.Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, Campbell M, Dickson P, Duh QY, Ehya H, Goldner WS, Guo T, Haymart M, Holt S, Hunt JP, Iagaru A, Kandeel F, Lamonica DM, Mandel S, Markovina S, McIver B, Raeburn CD, Rezaee R, Ridge JA, Roth MY, Scheri RP, Shah JP, Sipos JA, Sippel R, Sturgeon C, Wang TN, Wirth LJ, Wong RJ, Yeh M, Cassara CJ, Darlow S. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:925–951. doi: 10.6004/jnccn.2022.0040. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Xiao Z, Cheng L, Jian L. Iodine nutrition level and thyroid function in pregnant women in the Yongchuan district of Chongqing. J Clin Transl Res. 2022;8:516–522. [PMC free article] [PubMed] [Google Scholar]
- 9.Fan L, Du Y, Meng F, Liu L, Li M, Liu P, Sun D. How to decide the iodine content in salt for a country-China as an example. Nutrients. 2022;14:4606. doi: 10.3390/nu14214606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng LH, Chen CH, Liu Y, Liang XH, Zhou J, Xian J, Li L, Zhang J, Huang ZX, Qin YF. Epidemiological survey of the status of iodine nutrition and thyroid diseases in Guangxi, China. J Trace Elem Med Biol. 2022;70:126918. doi: 10.1016/j.jtemb.2021.126918. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Zhang F, Li Q, Feng C, Teng W. Iodine nutrition and papillary thyroid cancer. Front Nutr. 2022;9:1022650. doi: 10.3389/fnut.2022.1022650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen D, Singh S, Brito M. Thyroid, diet, and alternative approaches. J Clin Endocrinol Metab. 2022;107:2973–2981. doi: 10.1210/clinem/dgac473. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, Li J, Qian L, Cui L, Chen W, Wen C, Peng Y, Chen Q, Lu M, Chen M, Wu R, Zhou W, Xue E, Li Y, Yang L, Mi C, Zhang R, Wu G, Du G, Huang D, Zhan W Superficial Organ and Vascular Ultrasound Group of the Society of Ultrasound in Medicine of the Chinese Medical Association; Chinese Artificial Intelligence Alliance for Thyroid and Breast Ultrasound. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine. 2020;70:256–279. [Google Scholar]
- 14.Zhang X, Yuan N, Sun J, Zhao X, Du J, Nan M, Zhang Q, Ji L. Association between iodine nutritional status and adverse pregnancy outcomes in Beijing, China: a single-center cohort study. Biol Trace Elem Res. 2022;200:2620–2628. doi: 10.1007/s12011-021-02887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Can AS, Rehman A. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. Goiter. [Google Scholar]
- 16.Li Z, Zhang H, Chen W, Li H. Contrast-enhanced CT-based radiomics for the differentiation of nodular goiter from papillary thyroid carcinoma in thyroid nodules. Cancer Manag Res. 2022;14:1131–1140. doi: 10.2147/CMAR.S353877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MJ, Wang S, Zhang Q, He JL, Zhao HH, Hu WL, Huang F. Associations between environmental exposure to polybrominated diphenyl ethers and nodular goiter risk: a case-control study. Environ Res. 2022;212:113345. doi: 10.1016/j.envres.2022.113345. [DOI] [PubMed] [Google Scholar]
- 18.Agretti P, De Marco G, Ferrarini E, Di Cosmo C, Montanelli L, Bagattini B, Chiovato L, Tonacchera M. Gene expression profile in functioning and non-functioning nodules of autonomous multinodular goiter from an area of iodine deficiency: unexpected common characteristics between the two entities. J Endocrinol Invest. 2022;45:399–411. doi: 10.1007/s40618-021-01660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin L, Yu H, Mei J, Jia Z. Thyroid artery embolization for nodular goiter: the optimal candidates and techniques have yet to be determined. J Vasc Interv Radiol. 2022;33:200–201. doi: 10.1016/j.jvir.2021.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M, Tallini G, Mete O. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022;33:27–63. doi: 10.1007/s12022-022-09707-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Jiang Y, Song J, Liang H, Liu Y, Huang J, Yin P, Wu D, Zhang H, Liu X, Zhou D, Wei W, Lei L, Peng J, Zhang J. The risk of perchlorate and iodine on the incidence of thyroid tumors and nodular goiter: a case-control study in southeastern China. Environ Health. 2022;21:4. doi: 10.1186/s12940-021-00818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh G, Correa R. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. Diffuse toxic goiter. [Google Scholar]
- 23.Dos Santos PB, Gertrudes LN, Conceição FL, de Andrade BM, de Carvalho DP, Vaisman M, Teixeira PFDS. Effects of metformin on TSH levels and benign nodular goiter volume in patients without insulin resistance or iodine insufficiency. Front Endocrinol (Lausanne) 2019;10:465. doi: 10.3389/fendo.2019.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdulkhabirova FM, Bezlepkina OB, Brovin DN, Vadina TA, Melnichenko GA, Nagaeva EV, Nikankina LV, Peterkova VA, Platonova NM, Rybakova AA, Soldatova TV, Troshina EA, Shiryaeva TY. Clinical practice guidelines “Management of iodine deficiency disorders”. Probl Endokrinol (Mosk) 2021;67:10–25. doi: 10.14341/probl12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yildirim Simsir I, Cetinkalp S, Kabalak T. Review of factors contributing to nodular goiter and thyroid carcinoma. Med Princ Pract. 2020;29:1–5. doi: 10.1159/000503575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberstadt AE, Nelson NC, Claude AK, Refsal KR, Scott-Moncrieff JC, Petroff BK, Langlois DK. Radioactive iodine uptake in hyperthyroid cats after administration of recombinant human thyroid stimulating hormone. J Vet Intern Med. 2018;32:1891–1896. doi: 10.1111/jvim.15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benvenga S, Guarneri F. Homology of pendrin, sodium-iodide symporter and apical iodide transporter. Front Biosci (Landmark Ed) 2018;23:1864–1873. doi: 10.2741/4677. [DOI] [PubMed] [Google Scholar]
- 28.Huang H, Shi Y, Liang B, Cai H, Cai Q. Low iodine in the follicular lumen caused by cytoplasm mis-localization of sodium iodide symporter may induce nodular goiter. Biol Trace Elem Res. 2017;179:165–171. doi: 10.1007/s12011-017-0960-z. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Liang J, Lin Y, Yao R. Differential expression of the Na(+)/I(-) symporter protein in thyroid cancer and adjacent normal and nodular goiter tissues. Oncol Lett. 2013;5:368–372. doi: 10.3892/ol.2012.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.V S, M M, B M. The prevalence of thyroid diseases in pregnancy and it’s relation to iron deficiency - a hospital based study. J Assoc Physicians India. 2022;70:11–12. [PubMed] [Google Scholar]
- 31.Møllehave LT, Eliasen MH, Strēle I, Linneberg A, Moreno-Reyes R, Ivanova LB, Kusić Z, Erlund I, Ittermann T, Nagy EV, Gunnarsdottir I, Arbelle JE, Troen AM, Pīrāgs V, Dahl L, Hubalewska-Dydejczyk A, Trofimiuk-Müldner M, de Castro JJ, Marcelino M, Gaberšček S, Zaltel K, Puig-Domingo M, Vila L, Manousou S, Nyström HF, Zimmermann MB, Mullan KR, Woodside JV, Völzke H, Thuesen BH. Register-based information on thyroid diseases in Europe: lessons and results from the EUthyroid collaboration. Endocr Connect. 2022;11:e210525. doi: 10.1530/EC-21-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes MA, Adams CS, Bragg S, Christian N. Thyroid and parathyroid conditions: hyperthyroidism. FP Essent. 2022;514:11–17. [PubMed] [Google Scholar]
- 33.Miles EA, Vahlberg T, Calder PC, Houttu N, Pajunen L, Koivuniemi E, Mokkala K, Laitinen K. Iodine status in pregnant women and infants in Finland. Eur J Nutr. 2022;61:2919–2927. doi: 10.1007/s00394-022-02852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dardari D, Laborne FX, Tourte C, Henry E, Penfornis A. Evaluation of iodine supplementation in pregnant women with gestational diabetes: IODIAB study. Healthcare (Basel) 2022;10:2388. doi: 10.3390/healthcare10122388. [DOI] [PMC free article] [PubMed] [Google Scholar]


